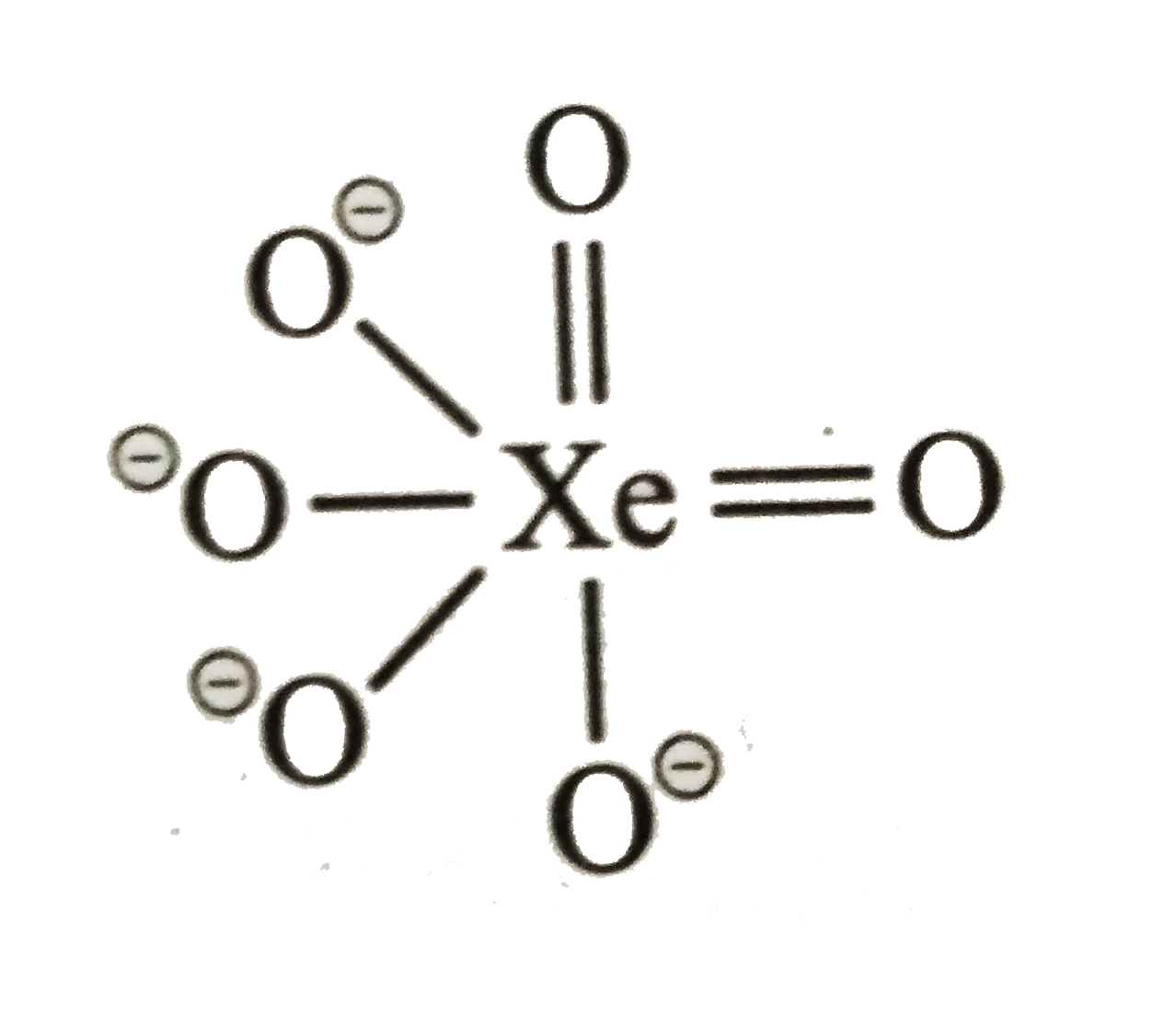A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
REDOX REACTIONS
CENGAGE CHEMISTRY|Exercise Exercises (Assertion-Reasoning)|15 VideosREDOX REACTIONS
CENGAGE CHEMISTRY|Exercise Exercises (Integers)|11 VideosREDOX REACTIONS
CENGAGE CHEMISTRY|Exercise Exercises (Multiple Correct)|32 VideosPURIFICATION OF ORGANIC COMPOUNDS AND QUALITATIVE AND QUANTITATIVE ANALYSIS
CENGAGE CHEMISTRY|Exercise Assertion Reasoning Type|5 VideosS-BLOCK GROUP 1 - ALKALI METALS
CENGAGE CHEMISTRY|Exercise Archives Subjective|8 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-REDOX REACTIONS-Exercises (Single Correct)
- Excess of KI reacts with CuSO(4) solution and Na(2)SO(3) solution is a...
Text Solution
|
- The oxidation number of S in H(2)SO(5) is
Text Solution
|
- The number of peroxide bonds in perxenate ion [XeO(6)]^(4-) is
Text Solution
|
- The oxidation number of Pr in Pr(6)O(11) is
Text Solution
|
- In which of the following is the highest oxidation state not possible?
Text Solution
|
- which of the following statements is not correct about the reaction gi...
Text Solution
|
- Which of the following is not a disproportionation reaction ?
Text Solution
|
- Which of the following is not an intramolecular redox reaction?
Text Solution
|
- In the equation N(2)^(ө)+H(2)OrarrNO(3)^(ө)+2H^(o+)+ne^(-) n stand...
Text Solution
|
- Which of the following is an intermolecular redox reaction?
Text Solution
|
- The oxidation state of A, B, and C in a compound are +2, +5, and -2, r...
Text Solution
|
- The number of electrons lost in the following change is Fe+H(2)Orarr...
Text Solution
|
- The oxidation number of Pt in [Pt (C(2)H(4))Cl(3))"]"^(ө) is
Text Solution
|
- The oxidation number of P in Mg(2)P(2)O(7) is
Text Solution
|
- The oxidation number of phosphorus in PO(4)^(3-), P(4)O(10), and P(2)O...
Text Solution
|
- which of the following leads to redox reaction ?
Text Solution
|
- The oxidation number of sulphur in Na(2)S(4)O(6) is
Text Solution
|
- The oxidiant state of iodine in H(4)IO(6)^(ө) is
Text Solution
|
- When iron is rusted, it is
Text Solution
|
- An element that never has a positive oxidation state in any of its com...
Text Solution
|