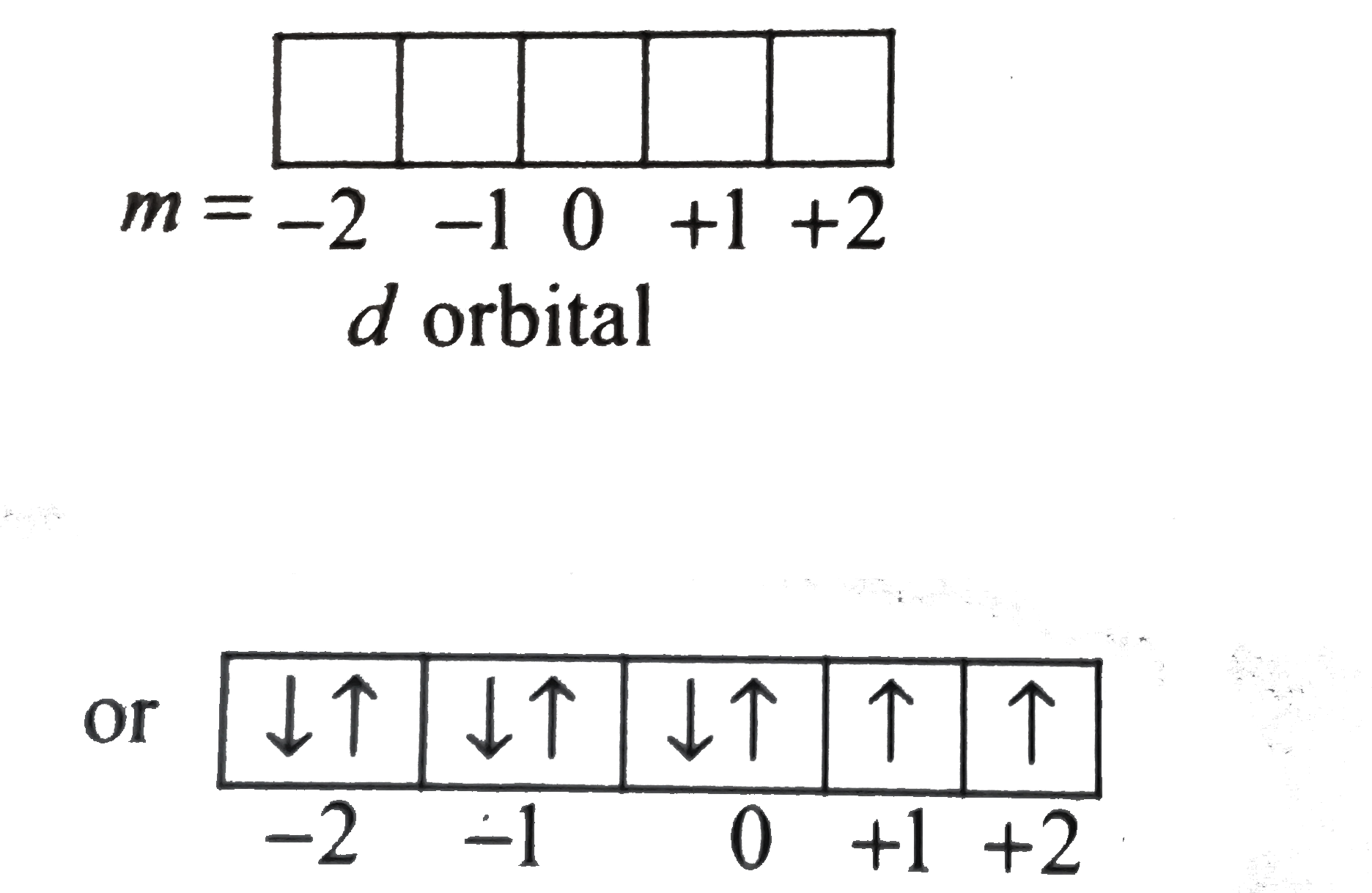Text Solution
Verified by Experts
Topper's Solved these Questions
ATOMIC STRUCTURE
CENGAGE CHEMISTRY|Exercise Exercises (Subjective)|52 VideosATOMIC STRUCTURE
CENGAGE CHEMISTRY|Exercise Exercises Linked Comprehension|50 VideosAPPENDIX - INORGANIC VOLUME 1
CENGAGE CHEMISTRY|Exercise chapter-7 Single correct answer|1 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
CENGAGE CHEMISTRY|Exercise Archives Subjective|15 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-ATOMIC STRUCTURE-Concept Applicationexercise(4.3)
- The quantum number of the last electron of an element are below pral...
Text Solution
|
- How many quantum number are needed in designate an orbital ? Name the...
Text Solution
|
- The principal quantum number of n of an atomic orbitals is 5 what are ...
Text Solution
|
- The azimathal quantum number l of an arbital is 3 what are the possi...
Text Solution
|
- What is the lowest value of n that allows g orbitals to exist?
Text Solution
|
- Given the notation for the sub-shell deotected by the following quant...
Text Solution
|
- How many electron on a fully filled l sub-shell have m(1) = 0 ?
Text Solution
|
- An electron is in one of this electrons
Text Solution
|
- If the largest value ofm(1) for an electron is + 3 in what type of su...
Text Solution
|
- Explain , giving reason , which of the following sets of quantum numbe...
Text Solution
|
- How many electron in an atom may have the following quantum number ? A...
Text Solution
|
- How many orbitals are possible in a. 4th energy level b. 5f sub-she...
Text Solution
|
- What are the possible value of m(1) for the different orbital of a....
Text Solution
|
- What is the shape 2s orbital .Give two9 point of difference between 1...
Text Solution
|
- a. How many sub-shell are associated with n = 4? b. How many electr...
Text Solution
|
- How many spectrical nodal syrface are there in a. a 3s orbital b. a...
Text Solution
|
- The principal quantum number representwsw
Text Solution
|
- The energy of an electron of 2p(1) orbital is
Text Solution
|
- The orbital angular momentum of an electron of an electron in 2s orbit...
Text Solution
|
- The number of nodals plates of zeroelectron density in the d(xy) orbit...
Text Solution
|