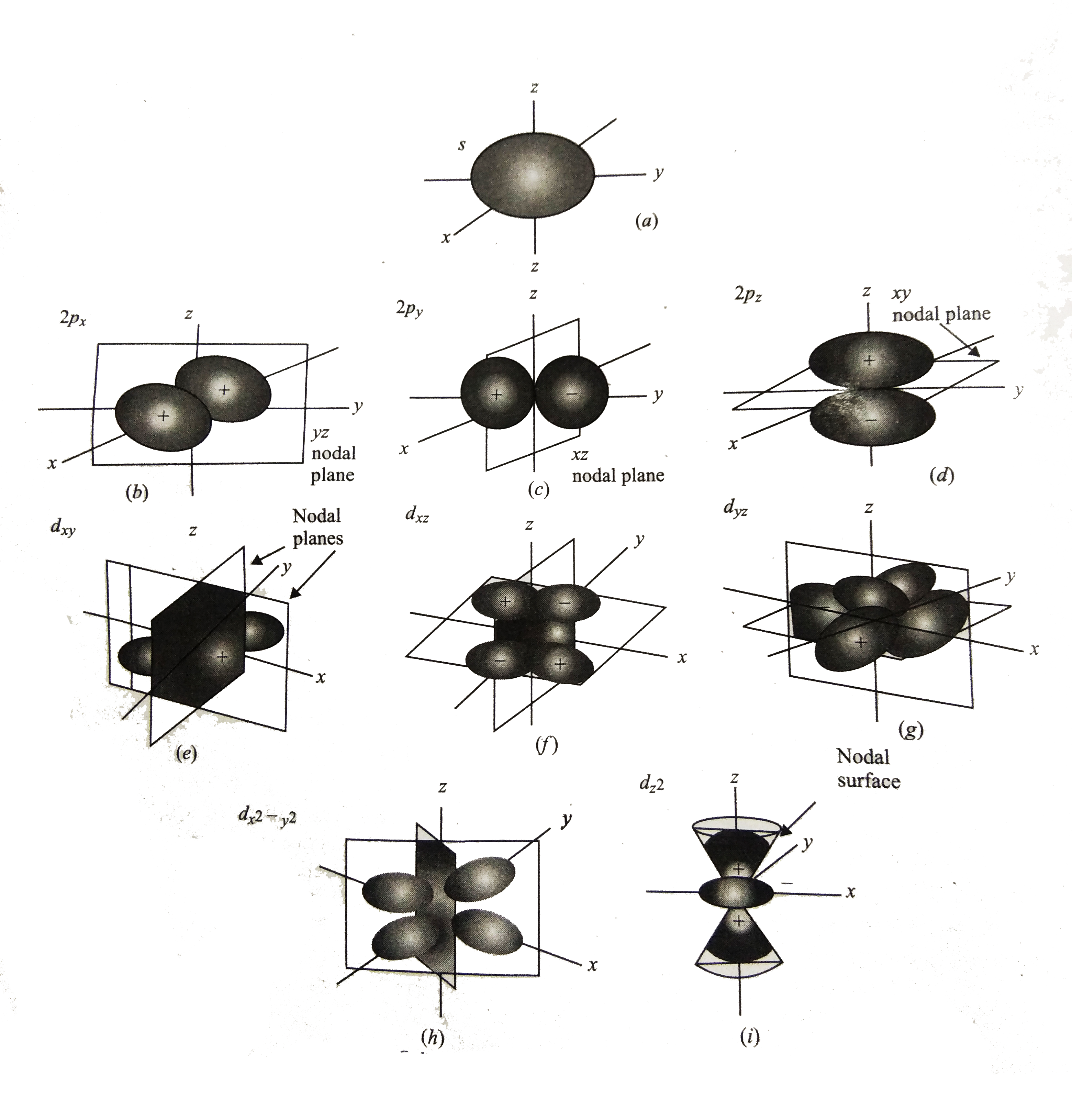
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ATOMIC STRUCTURE
CENGAGE CHEMISTRY|Exercise Exercises Assertion And Reason|21 VideosATOMIC STRUCTURE
CENGAGE CHEMISTRY|Exercise Exercises Integer|11 VideosATOMIC STRUCTURE
CENGAGE CHEMISTRY|Exercise Exercises Multiple Correct|45 VideosAPPENDIX - INORGANIC VOLUME 1
CENGAGE CHEMISTRY|Exercise chapter-7 Single correct answer|1 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
CENGAGE CHEMISTRY|Exercise Archives Subjective|15 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-ATOMIC STRUCTURE-Exercises Single Correct
- The correct set of quantum number for the unpaired electron of chlorin...
Text Solution
|
- The orbital diagram in which the Aufhan principle is violated is
Text Solution
|
- The first loinsatisation in electron volts of nitrogen and oxygen ato...
Text Solution
|
- Atomic radil of flaorine and meon in Angstrom units are respectively ...
Text Solution
|
- The ratio of energy of photon of lambda = 2000 Å to that of lambda = 4...
Text Solution
|
- The sum of the number of neutrons and proton in the isotope of hydroge...
Text Solution
|
- The radius of an atomic nucleus is of the order of
Text Solution
|
- Which of the following is true ?
Text Solution
|
- Which of the following is true ?
Text Solution
|
- Which of the following is true ?
Text Solution
|
- Which of the following is false?
Text Solution
|
- Which of the following is true ?
Text Solution
|
- Which of the following is true ?
Text Solution
|
- Which of the following is false?
Text Solution
|
- Which of the following is false?
Text Solution
|
- Which of the following is true ?
Text Solution
|
- Which of the following is false?
Text Solution
|
- Which of the following is false?
Text Solution
|
- Which of the following is true ?
Text Solution
|
- Which of the following is false?
Text Solution
|