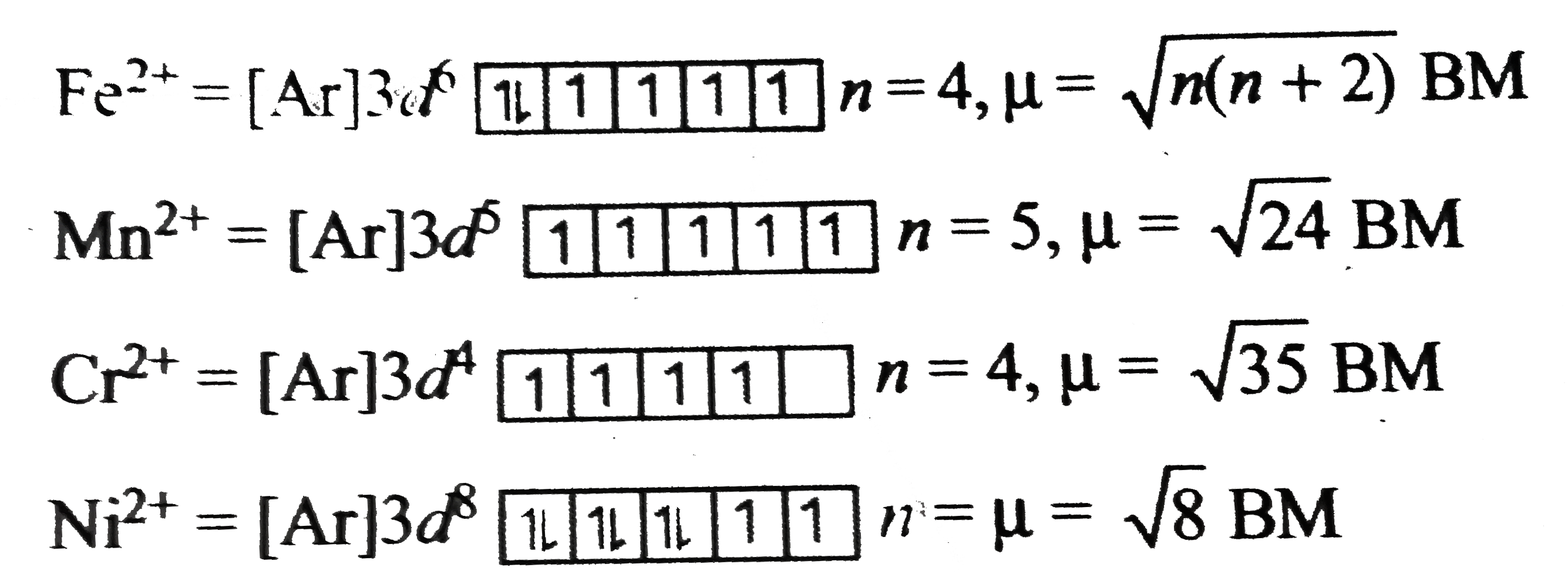A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ATOMIC STRUCTURE
CENGAGE CHEMISTRY|Exercise Exercises Fill In The Balnks|56 VideosATOMIC STRUCTURE
CENGAGE CHEMISTRY|Exercise Exercises True And False|30 VideosATOMIC STRUCTURE
CENGAGE CHEMISTRY|Exercise Exercises Assertion And Reason|21 VideosAPPENDIX - INORGANIC VOLUME 1
CENGAGE CHEMISTRY|Exercise chapter-7 Single correct answer|1 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
CENGAGE CHEMISTRY|Exercise Archives Subjective|15 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-ATOMIC STRUCTURE-Exercises Integer
- What is the total number of electrons atlest same quantum number for B...
Text Solution
|
- The magnitude of an orbital angular momentum vector of an electron is ...
Text Solution
|
- A certain transition is H spectrum from an excited state to the gr...
Text Solution
|
- The ucertianity in the possition of an electron is equal to its de Vbr...
Text Solution
|
- The sum of all the quantum number of helium atomm is
Text Solution
|
- The maximum number of dectrens that can be accomodeated in an orbital ...
Text Solution
|
- The orbital angular mometum quantum number of the state s(2) is
Text Solution
|
- How many of the following are possible 1p,2s,3p,3f,3d
Text Solution
|
- How many of the following ions have the same magnetic moments ? Fe^(2...
Text Solution
|
- The number of nodes in 3p orbital
Text Solution
|
- If each hydrogen atom in the ground state 1.0 mol of H atom are excit...
Text Solution
|