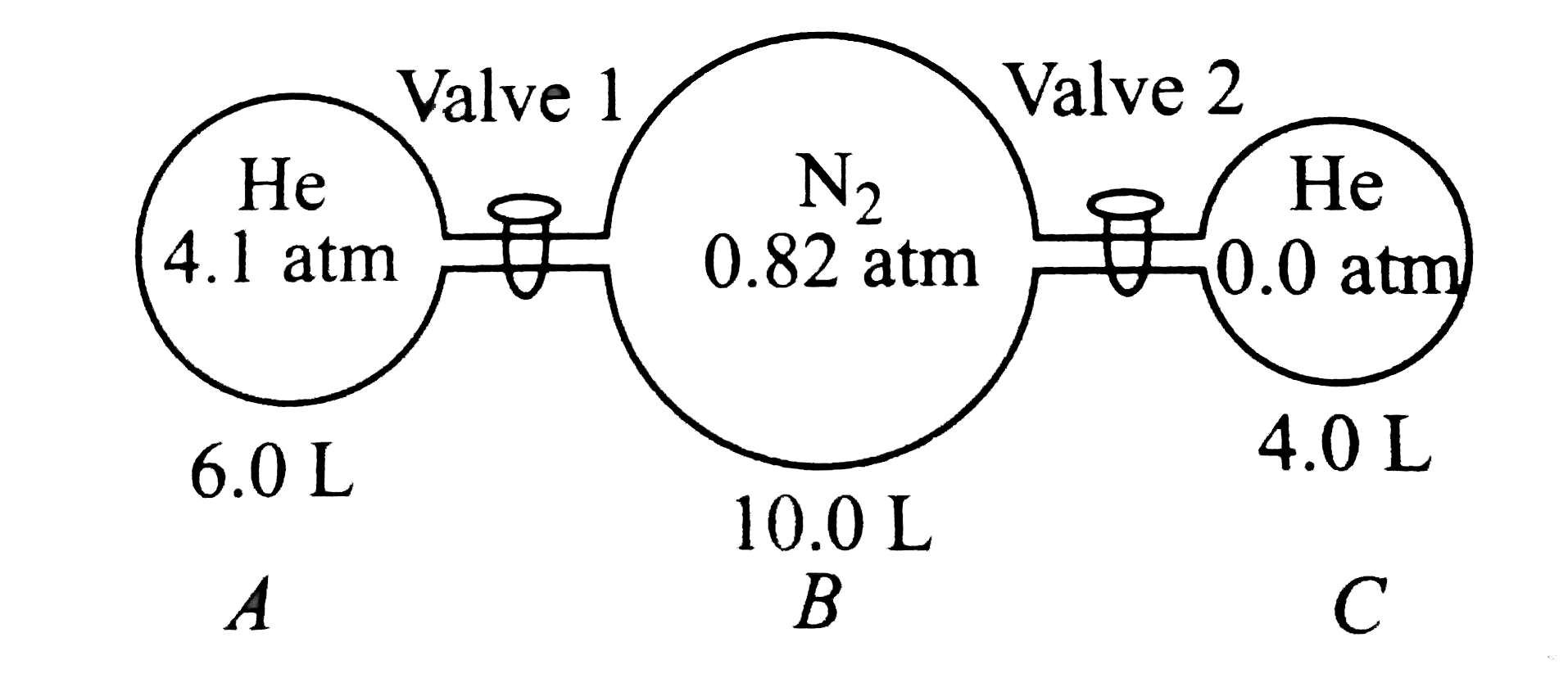A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
STATES OF MATTER
CENGAGE CHEMISTRY|Exercise Exercises (Multiple Correcttype)|32 VideosSTATES OF MATTER
CENGAGE CHEMISTRY|Exercise Exercises (Single Correct)|85 VideosSTATES OF MATTER
CENGAGE CHEMISTRY|Exercise Exercises|21 VideosSOME BASIC CONCEPTS AND MOLE CONCEPT
CENGAGE CHEMISTRY|Exercise Archives Subjective|11 VideosSTOICHIOMETRY
CENGAGE CHEMISTRY|Exercise Archives Subjective|33 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-STATES OF MATTER-Exercises (Linked Comprehensive)
- The figure given below shows three glass chambers that are connected b...
Text Solution
|
- The figure given below shows three glass chambers that are connected b...
Text Solution
|
- The figure given below shows three glass chambers that are connected b...
Text Solution
|
- The distribution of the molecular velocities of gas molecules at any t...
Text Solution
|
- The distribution of the molecular velocities of gas molecules at any t...
Text Solution
|
- The distribution of the molecular velocities of gas molecules at any t...
Text Solution
|
- Two flasks A and B have equal volume. A is maintained at 300 K and B a...
Text Solution
|
- Two flasks A and B have equal volume. A is maintained at 300 K and B a...
Text Solution
|
- Two flasks A and B have equal volume. A is maintained at 300 K and B a...
Text Solution
|
- Two flasks A and B have equal volume. A is maintained at 300 K and B a...
Text Solution
|
- Two flasks A and B have equal volume. A is maintained at 300 K and B a...
Text Solution
|
- Two flasks A and B have equal volume. A is maintained at 300 K and B a...
Text Solution
|
- The van der Waals constant for gases A, B, and C are as follows A...
Text Solution
|
- The van der Waals constant for gases A, B, and C are as follows A...
Text Solution
|
- The van der Waals constant for gases A, B, and C are as follows A...
Text Solution
|
- For the given ideal gas equation PV=nRT, answer the following question...
Text Solution
|
- For the given ideal gas equation PV=nRT, answer the following question...
Text Solution
|
- For the given ideal gas equation PV=nRT, answer the following question...
Text Solution
|
- For the given ideal gas equation PV=nRT, answer the following question...
Text Solution
|
- For the given ideal gas equation PV=nRT, answer the following question...
Text Solution
|