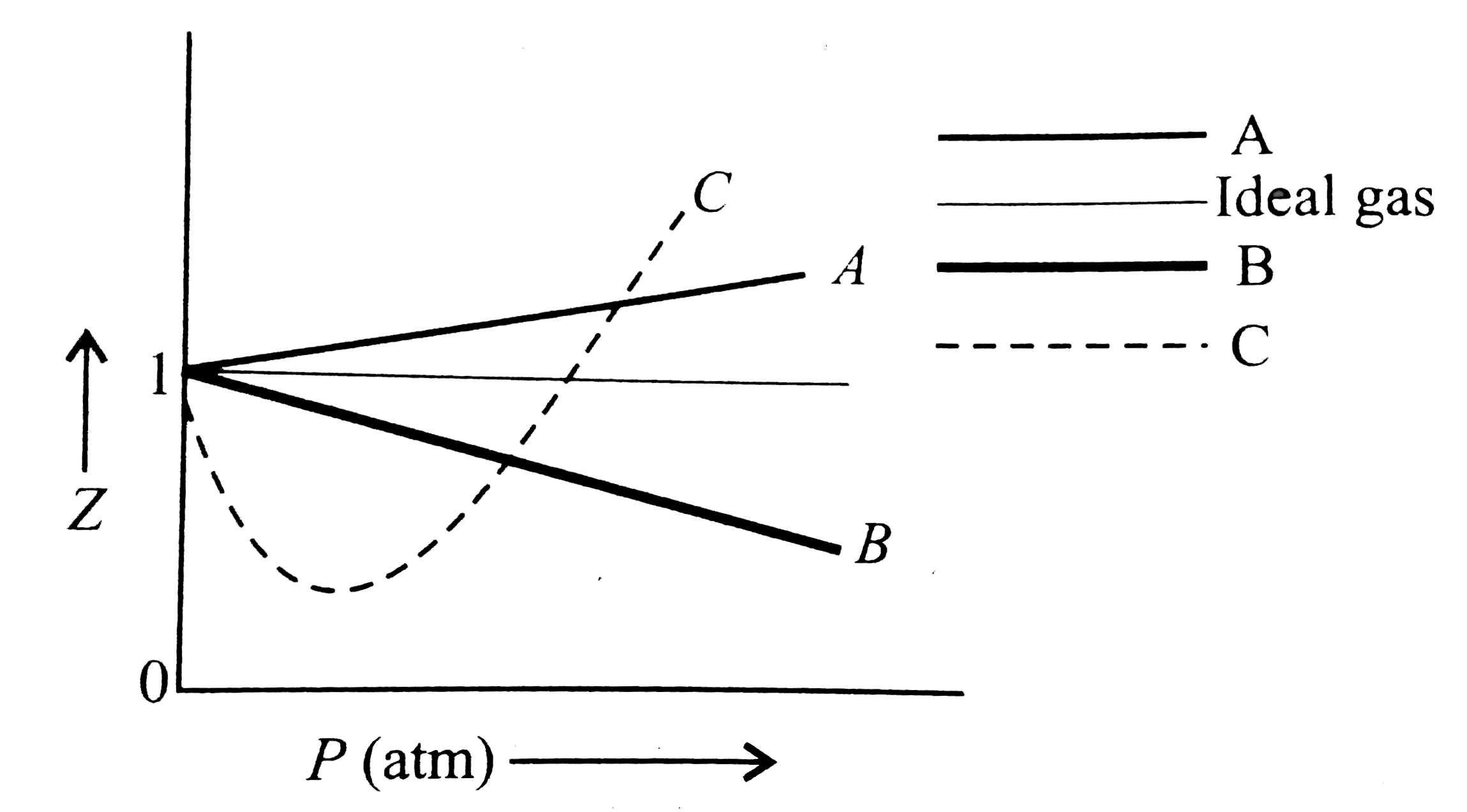
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
STATES OF MATTER
CENGAGE CHEMISTRY|Exercise Archives (Single Correct)|32 VideosSTATES OF MATTER
CENGAGE CHEMISTRY|Exercise Archives ( Assertion-Reasoning)|2 VideosSTATES OF MATTER
CENGAGE CHEMISTRY|Exercise Exercises (Integer)|10 VideosSOME BASIC CONCEPTS AND MOLE CONCEPT
CENGAGE CHEMISTRY|Exercise Archives Subjective|11 VideosSTOICHIOMETRY
CENGAGE CHEMISTRY|Exercise Archives Subjective|33 Videos
Similar Questions
Explore conceptually related problems