Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-THERMODYNAMICS-Archives (Subjective)
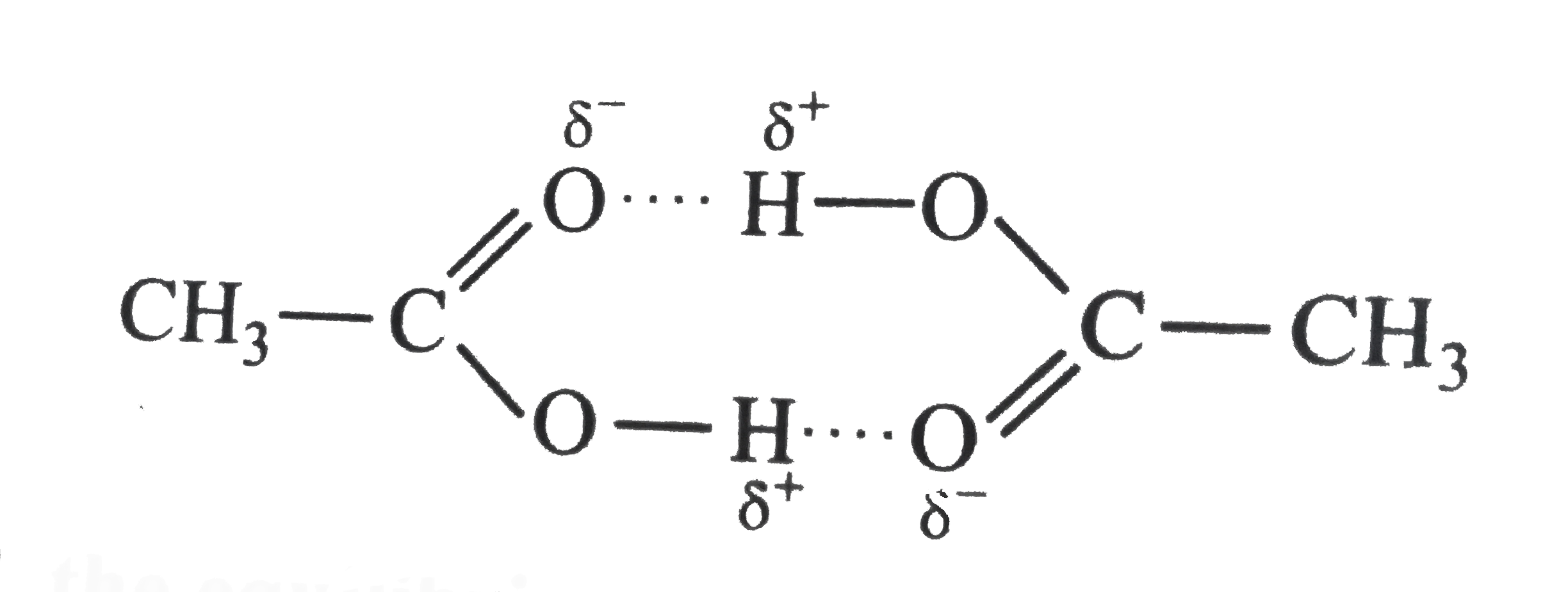
- Acetoc acid CH(3)COOH can form a dimer (CH(3)COOH)(2) in the gas phase...
Text Solution
|
- The enthalpies for the following reactions (DeltaH^(Theta)) at 25^(@)C...
Text Solution
|
- The standared enthalpies of formation at 298K for C C1(g), H(2)O(g), C...
Text Solution
|
- Given that: i. C(s) + O(2)(g) rarr CO(2)(g) , DeltaH =- 94.05 kcal ...
Text Solution
|
- The following statements is true only under some specific conditions. ...
Text Solution
|
- The bond dissociation energies of gaseous H(2),C1(2), and HC1 are 100,...
Text Solution
|
- The standard molar heats of formation of ethane, carbon dioxide, and l...
Text Solution
|
- An intimate mixture of ferric oxide and aluminium is used as solid fue...
Text Solution
|
- The standard ethelpy of combustion at 25^(@)C of hydrogen, cyclohexene...
Text Solution
|
- Using the data ( all vaues in kcal mol^(-1) at 25^(@)C) given below, c...
Text Solution
|
- Determine enthalpy change for, C(3)H(8(g))+H(2(g))rarr C(2)H(6(g))+C...
Text Solution
|
- Compute the heat of formation of liquie methyl alcohol is kilojoule pe...
Text Solution
|
- From the following data, calculate the enthalpy change for the combust...
Text Solution
|
- The standard heat of formation values of SF(6)(g), S(g), and F(g) are ...
Text Solution
|
- Show that the reaction CO(g) +(1//2)O(2)(g) rarr CO(2)(g) at 300K ...
Text Solution
|
- A sample of argon gas at 1atm pressure and 27^(@)C expands reversibly ...
Text Solution
|
- Diborane is a potential rocket fuel that undergoes combustion accordin...
Text Solution
|
- The standard potential of the following cell is 0.23V at 15^(@)C and 0...
Text Solution
|
- When 1pentyne (A) is treated with 4N alcoholic KOH at 175^(@)C, it is ...
Text Solution
|
- Two moles of a perfect gas undergo the following processes: a. A rev...
Text Solution
|
- C(v) values of He is always (3R)/(2) but C(v) values of H(2) is (3R)/(...
Text Solution
|