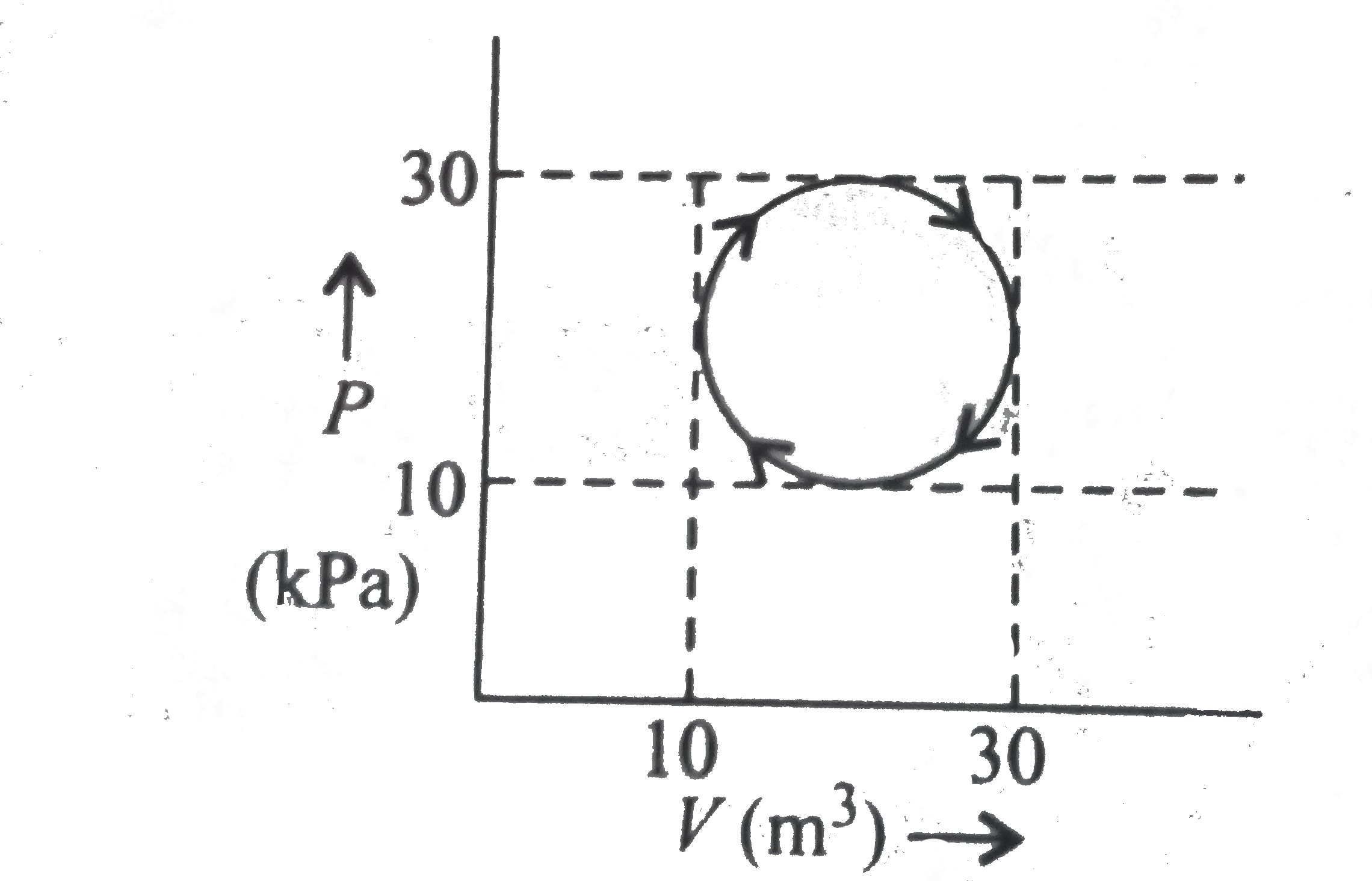
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
THERMODYNAMICS
CENGAGE CHEMISTRY|Exercise Exercises (Single Correct)|62 VideosTHERMODYNAMICS
CENGAGE CHEMISTRY|Exercise Exercises (Assertion-Reasoning)|23 VideosTHERMODYNAMICS
CENGAGE CHEMISTRY|Exercise Exercises (Linked Comprehension)|74 VideosSTOICHIOMETRY
CENGAGE CHEMISTRY|Exercise Archives Subjective|33 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-THERMODYNAMICS-Exercises (Multiple Correct)
- Which of the following are intensive properties?
Text Solution
|
- Which of the following are extensive properties ?
Text Solution
|
- Which one is not correct for a cyclic process as shown in the figure ?
Text Solution
|
- If w(1).w(2),w(3) and w(4) are work done in isothermal, adiabatic, iso...
Text Solution
|
- Average value of poisson's ratio for a mixture of 2 mole of each gas A...
Text Solution
|
- A reaction attains equilibrium state under standard conditions, then:
Text Solution
|
- The poisson's ratio for O(2) is 1.4. Which of the following are correc...
Text Solution
|
- Select the correct statements.
Text Solution
|
- Select the correct statements for the equilibrium under standard condi...
Text Solution
|
- Which is intensive property ?
Text Solution
|
- Which of the followinf statements are correct?
Text Solution
|
- Following enthalpy changes are given: alpha-Dglucose (s) rarr alpha...
Text Solution
|
- If x and y are arbitrary extensive variables, then
Text Solution
|
- If x and y are arbitrary intensive variables, then
Text Solution
|
- For which process does DeltaU = 0 holds true?
Text Solution
|
- Which is correct about DeltaG?
Text Solution
|
- Which is not correct relationship between DeltaG^(Theta) and equilibri...
Text Solution
|
- Which is not correct relationship?
Text Solution
|
- Which of the following are endothermic processes?
Text Solution
|
- For an idela gas undergoing isothermal irreversible expansion
Text Solution
|