A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL EQUILIBRIUM
CENGAGE CHEMISTRY|Exercise Exercises (Multiple Correct)|26 VideosCHEMICAL EQUILIBRIUM
CENGAGE CHEMISTRY|Exercise Exercises (Single Correct)|58 VideosCHEMICAL EQUILIBRIUM
CENGAGE CHEMISTRY|Exercise Exercises (Subjective)|46 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
CENGAGE CHEMISTRY|Exercise Archives Subjective|15 VideosCLASSIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDS
CENGAGE CHEMISTRY|Exercise Analytical and Descriptive Type|3 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-CHEMICAL EQUILIBRIUM-Exercises (Linked Comprehensive)
- One "mole" of NH(4)HS(s) was allowed to decompose in a 1-L container a...
Text Solution
|
- One "mole" of NH(4)HS(s) was allowed to decompose in a 1-L container a...
Text Solution
|
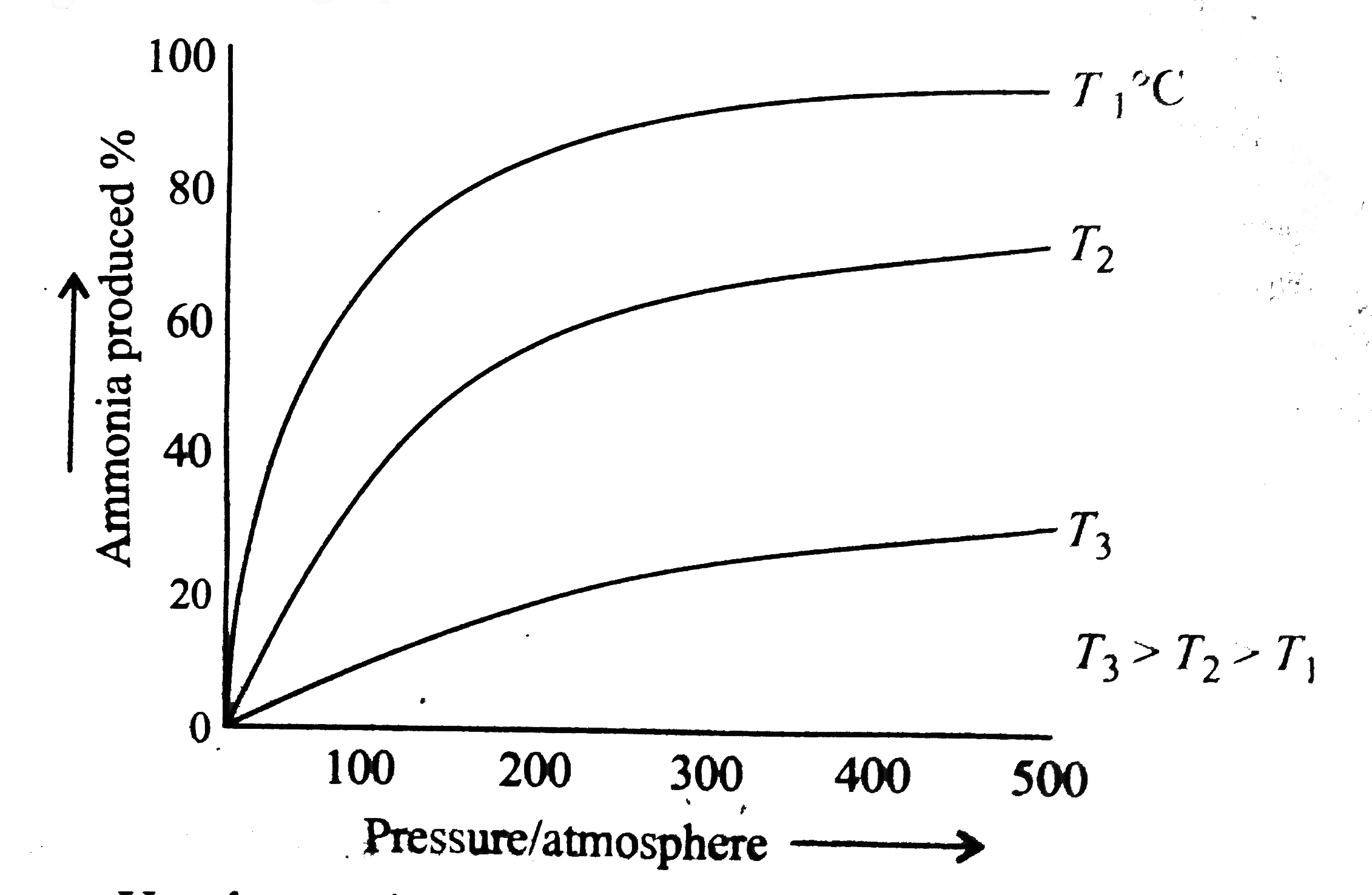
- The persentage of ammonia produced from nitrogen and hydrogen under co...
Text Solution
|
- The persentage of ammonia produced from nitrogen and hydrogen under co...
Text Solution
|
- The persentage of ammonia produced from nitrogen and hydrogen under co...
Text Solution
|
- The synthesis of ammonia is given as: N(2)(g)+3H(2)(g) hArr 2NH(3)(g...
Text Solution
|
- The synthesis of ammonia is given as: N(2)(g)+3H(2)(g) hArr 2NH(3)(g...
Text Solution
|
- The synthesis of ammonia is given as: N(2)(g)+3H(2)(g) hArr 2NH(3)(g...
Text Solution
|
- The synthesis of ammonia is given as: N(2)(g)+3H(2)(g) hArr 2NH(3)(g...
Text Solution
|
- The synthesis of ammonia is given as: N(2)(g)+3H(2)(g) hArr 2NH(3)(g...
Text Solution
|
- Phosphorous pentachloride when heated in a sealed tube at 700 K it und...
Text Solution
|
- Phosphorous pentachloride when heated in a sealed tube at 700 K it und...
Text Solution
|
- Phosphorous pentachloride when heated in a sealed tube at 700 K it und...
Text Solution
|
- Phosphorous pentachloride when heated in a sealed tube at 700 K it und...
Text Solution
|
- Phosphorous pentachloride when heated in a sealed tube at 700 K it und...
Text Solution
|
- Decomposition of ammonium chloride is an endothermic reaction. The equ...
Text Solution
|
- Decomposition of ammonium chloride is an endothermic reaction. The equ...
Text Solution
|
- Decomposition of ammonium chloride is an endothermic reaction. The equ...
Text Solution
|
- Decomposition of ammonium chloride is an endothermic reaction. The equ...
Text Solution
|
- Decomposition of ammonium chloride is an endothermic reaction. The equ...
Text Solution
|