A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
IONIC EQUILIBRIUM
CENGAGE CHEMISTRY|Exercise Ex 8.2|27 VideosIONIC EQUILIBRIUM
CENGAGE CHEMISTRY|Exercise Ex 8.3|38 VideosIONIC EQUILIBRIUM
CENGAGE CHEMISTRY|Exercise Archives Subjective|28 VideosHYDROGEN, WATER AND HYDROGEN PEROXIDE
CENGAGE CHEMISTRY|Exercise Subjective Archive (Subjective)|3 VideosISOMERISM
CENGAGE CHEMISTRY|Exercise Assertion-Reasoning Type|1 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-IONIC EQUILIBRIUM-Ex 8.1
- 100mL of HC1 gas at 25^(@)C and 740mm pressure is dissolved in 1L of H...
Text Solution
|
- Calculate [C1^(Theta)], [Na^(o+)], [H^(o+)], [overset(Theta)OH], and t...
Text Solution
|
- Calculate the pH of solution obtained by mixing 10 ml of 0.1 M HCl and...
Text Solution
|
- Calculate the pH of a solution which contains 100mL of 0.1 M HC1 and 9...
Text Solution
|
- Calculate the [H^(o+)] and [overset(Theta)OH] of 0.0315g of HNO(3) in ...
Text Solution
|
- 25.0 mL of 0.1M NaOh is titred with 0.1M HC1. Calculate pH when: i. ...
Text Solution
|
- The conjugate acid of overset(Theta)NH(2) is
Text Solution
|
- Which solutionwill have pH closer to 1.0 ?
Text Solution
|
- An acid solution of pH =6 is diluted 100 times. The pH of solution bec...
Text Solution
|
- The number of H^(o+) ions present in 1mL of solution having pH = 13 is
Text Solution
|
- Equal volumes of two solutions of Hc1 are mixed. One solution has a pH...
Text Solution
|
- For pure water,
Text Solution
|
- The pH of a solution increased from 3 to 6. Its [H^(o+)] will be
Text Solution
|
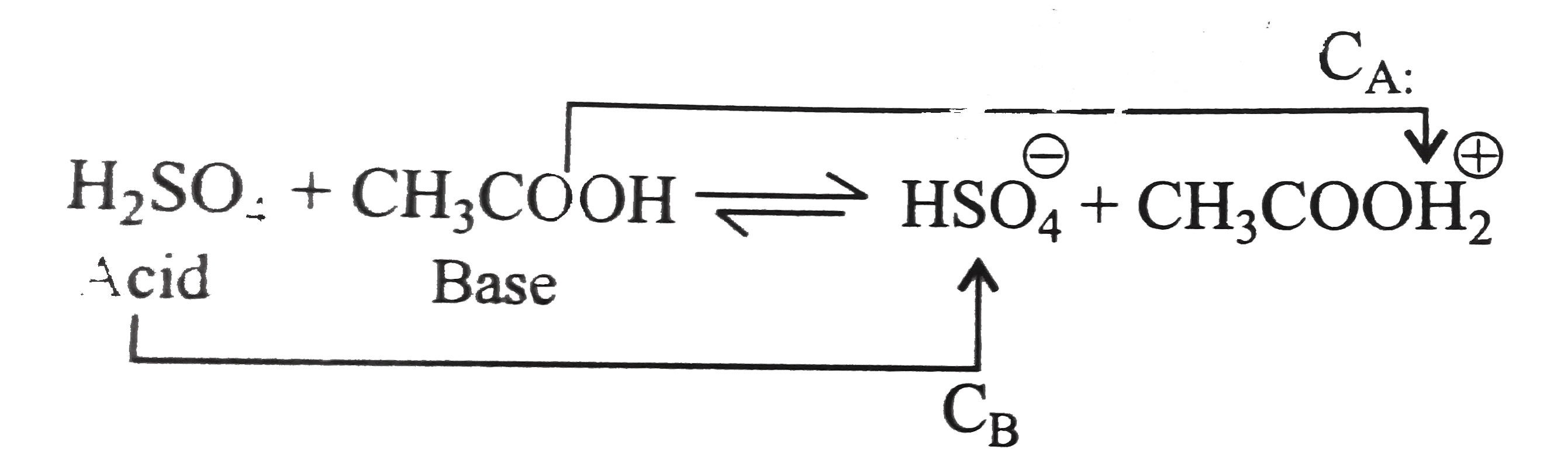
- The following equilibria is established when H(2)SO(4) is dissolved in...
Text Solution
|
- Which of the following consitute a set of atomspheric species?
Text Solution
|
- One litre of 0.5 M KC1 is electrolysed by passing 9650 coulombs of ele...
Text Solution
|
- pH of a solution made by mixing 200mL of 0.0657M NaOH, 140 mL of 0.107...
Text Solution
|
- When one drop of a concentrated HC1 is added to 1L of pure water at 25...
Text Solution
|