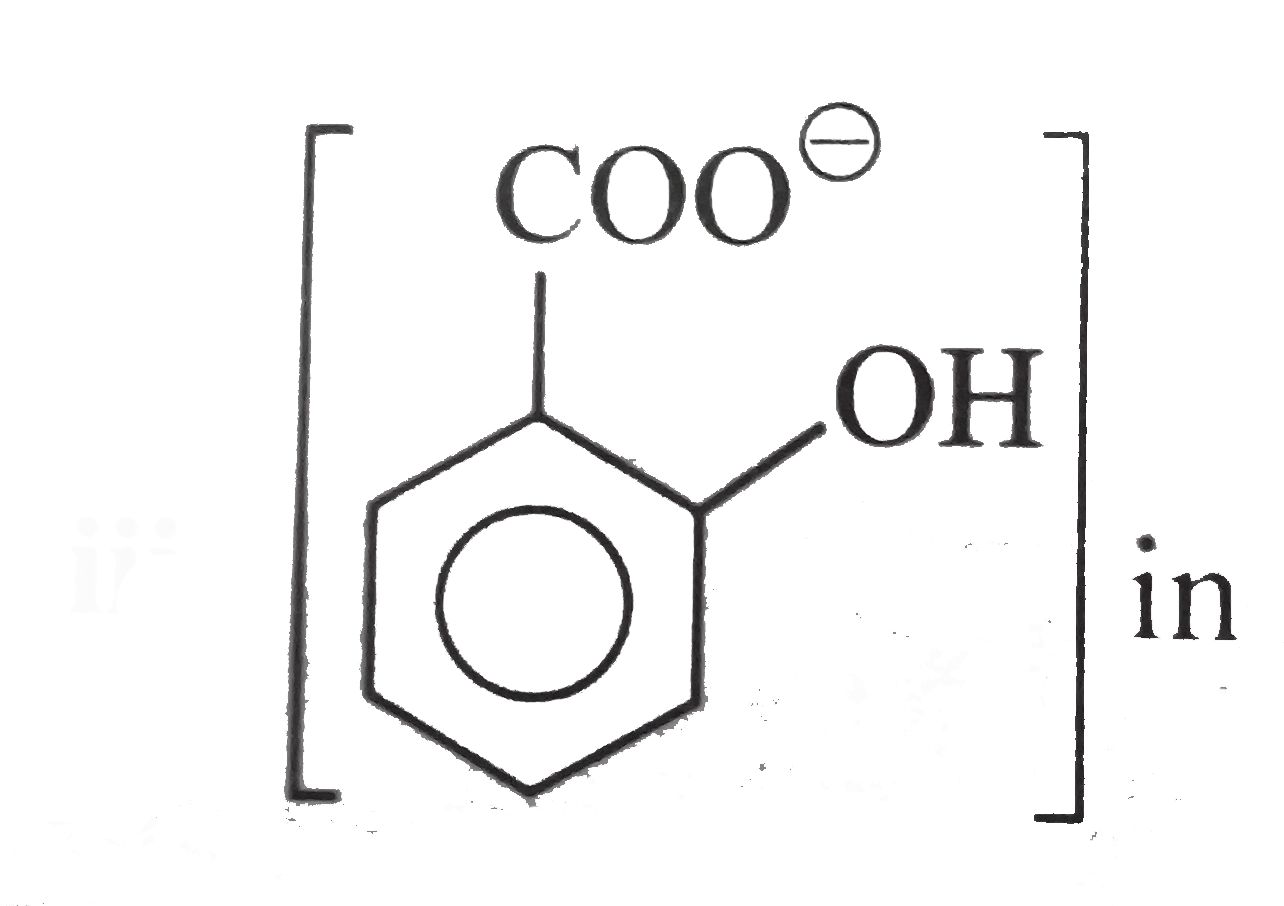
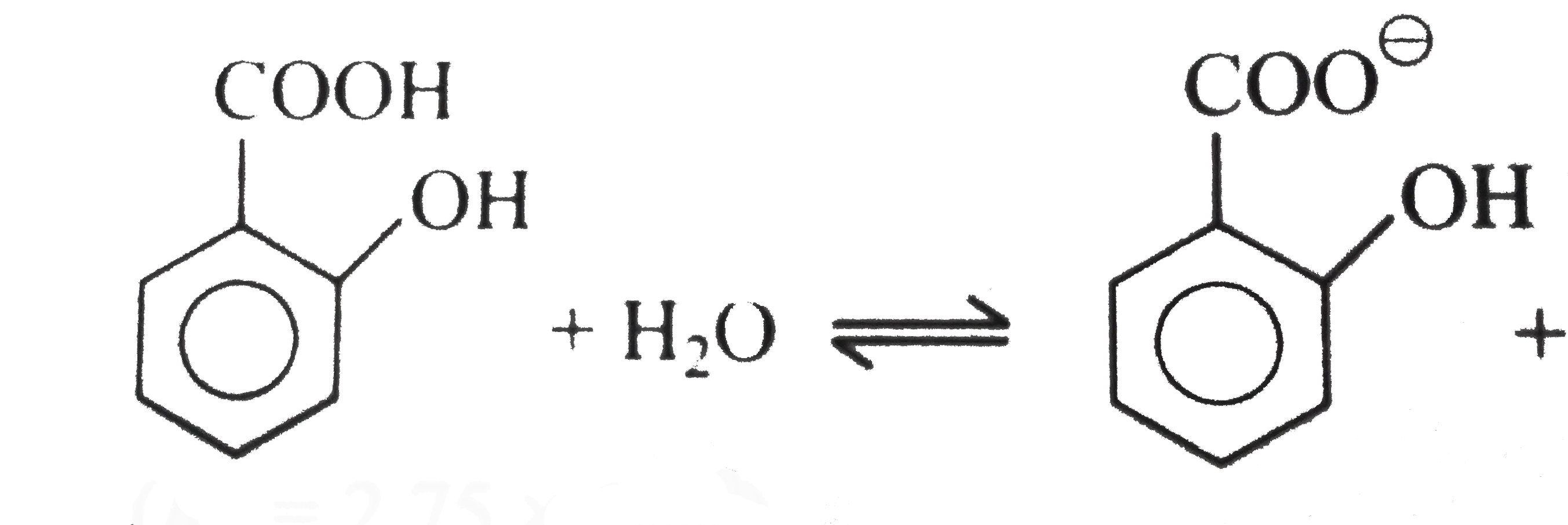Text Solution
Verified by Experts
Topper's Solved these Questions
IONIC EQUILIBRIUM
CENGAGE CHEMISTRY|Exercise Exercises Subjective (Buffer Solutions)|2 VideosIONIC EQUILIBRIUM
CENGAGE CHEMISTRY|Exercise Exercises Subjective (Hydrolysis Of Salt)|2 VideosIONIC EQUILIBRIUM
CENGAGE CHEMISTRY|Exercise Ex 8.5|6 VideosHYDROGEN, WATER AND HYDROGEN PEROXIDE
CENGAGE CHEMISTRY|Exercise Subjective Archive (Subjective)|3 VideosISOMERISM
CENGAGE CHEMISTRY|Exercise Assertion-Reasoning Type|1 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-IONIC EQUILIBRIUM-Exercises Subjective (Weak Acid And Weak Bases)
- a. Distinguish between acid strength and acid concentration. b. Dist...
Text Solution
|
- a. Write an equilibriu equation for a solution containing CH(3)COOH an...
Text Solution
|
- Which of the reagents listed below could be added to water to make 0.1...
Text Solution
|
- Saccharin (K(a)=2xx10^(-12)) is a weak acid represented by formula HSa...
Text Solution
|
- Accety1 salocylic acid (aspirin) ionises in water as: (K(a) = 2.7...
Text Solution
|
- Calculate the [CH(2)FCOOH] (fluoroacetic acid) which is required to ge...
Text Solution
|
- Calculate the dissociation constant of NH(4)OH at 298k, if DeltaH^(The...
Text Solution
|
- Determine the dergee of dissociation of 0.05 M NH(3) at 25^(@)C in a s...
Text Solution
|
- In the eqantitative analysis Bi^(3+) is detected precipitation of [BiO...
Text Solution
|
- Calculate the [overset(Theta)OH] of [NH(2)C(2)H(4)NH(3)]^(o+) and [H(3...
Text Solution
|
- Calculate pH of a. 0.002 N CH(3) COOH having 2.3% dissociation. b....
Text Solution
|
- Calculate [H^(o+)] and [CHC1(2)COO^(Theta)] in a solution that is 0.01...
Text Solution
|
- A solution contains 0.09 HC1, 0.09 M CHC1(2)COOH, and 0.1M CH(3)COOH. ...
Text Solution
|
- What is the concentration of CH(3)COOH which can be added to 0.5M HCOO...
Text Solution
|
- What are [H^(o+)], [A^(Theta), and [B^(Theta)] in a solution that is 0...
Text Solution
|