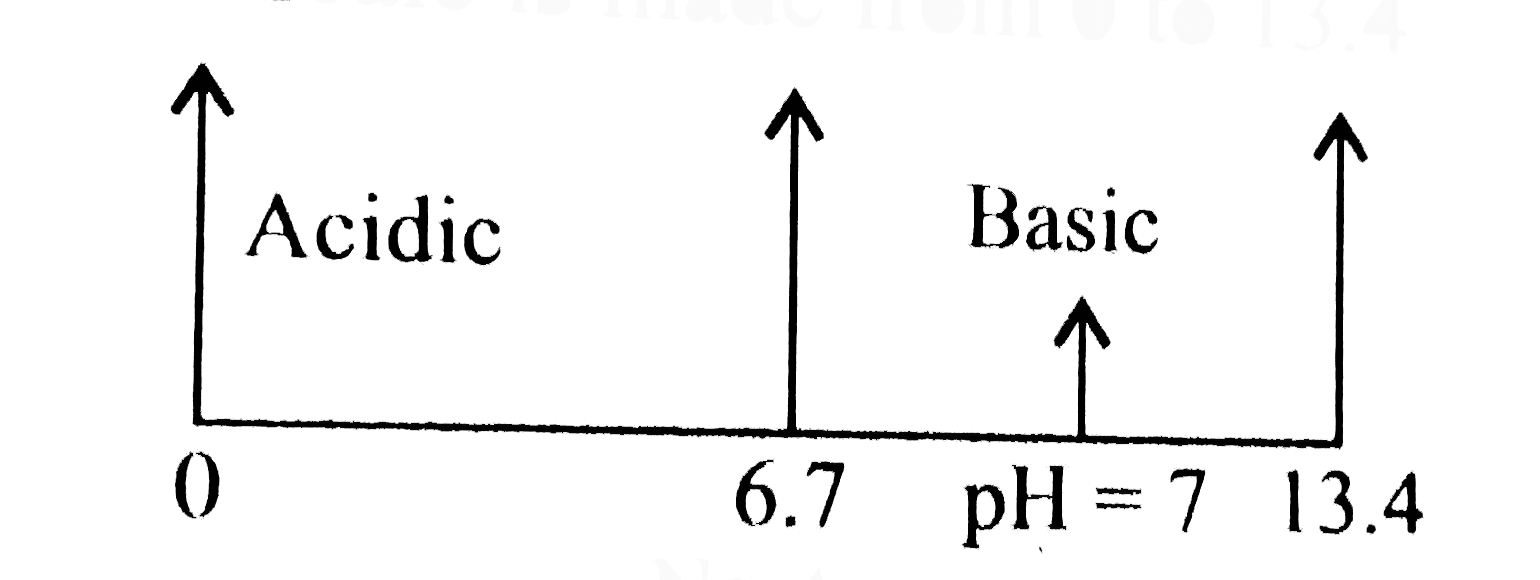A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
IONIC EQUILIBRIUM
CENGAGE CHEMISTRY|Exercise Exercises Assertion-Reasoning|36 VideosIONIC EQUILIBRIUM
CENGAGE CHEMISTRY|Exercise Exercises Integer|10 VideosIONIC EQUILIBRIUM
CENGAGE CHEMISTRY|Exercise Exercises Multiple Correct|33 VideosHYDROGEN, WATER AND HYDROGEN PEROXIDE
CENGAGE CHEMISTRY|Exercise Subjective Archive (Subjective)|3 VideosISOMERISM
CENGAGE CHEMISTRY|Exercise Assertion-Reasoning Type|1 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-IONIC EQUILIBRIUM-Exercises Single Correct
- One of the following is a Bronsted acid but not a Bronsted base:
Text Solution
|
- In the third group of qualitive analysis, the precipitating reagent is...
Text Solution
|
- At a certain temperature the value of pK(w) is 13.4 and the measured p...
Text Solution
|
- When 2mol of HCI is added to 1L of an acidic buffer, its pH changes fr...
Text Solution
|
- Let the solubilities of AgCI in H(2)O, and in 0.01M CaCI(2), 0.01M NaC...
Text Solution
|
- Which of the following salts will not undergo hydrolysis in water?
Text Solution
|
- Which of the following salts will not change the pH of pure water on d...
Text Solution
|
- A salt X is dissolved in water having pH =7. The resulting solution ha...
Text Solution
|
- The pH of a solution 7.00. To this solution, sufficient base is added ...
Text Solution
|
- Assuming H(2)SO(4) to be completely ionised the pH of a 0.05M aqueous ...
Text Solution
|
- A solution has pOH equal to 13 at 298 K. The solution will be
Text Solution
|
- If ammonia is added to pure water, the concentration of a chemical spe...
Text Solution
|
- The pH of a dilute solution of acetic acid wea found to be 4.3. The ad...
Text Solution
|
- Which of the following can act both as a Bronsted acid and a Bronsted...
Text Solution
|
- Which of the following is a Lewis base?
Text Solution
|
- Which of the following is not a Lewis base?
Text Solution
|
- Conjugate base of overset(Theta)OH is
Text Solution
|
- Which of the following will have the largest pH?
Text Solution
|
- Which one of following will have the largest pH?
Text Solution
|
- When 20 mL of M//20NaOH is added to 10mL of M//10 HCI, the resulting s...
Text Solution
|