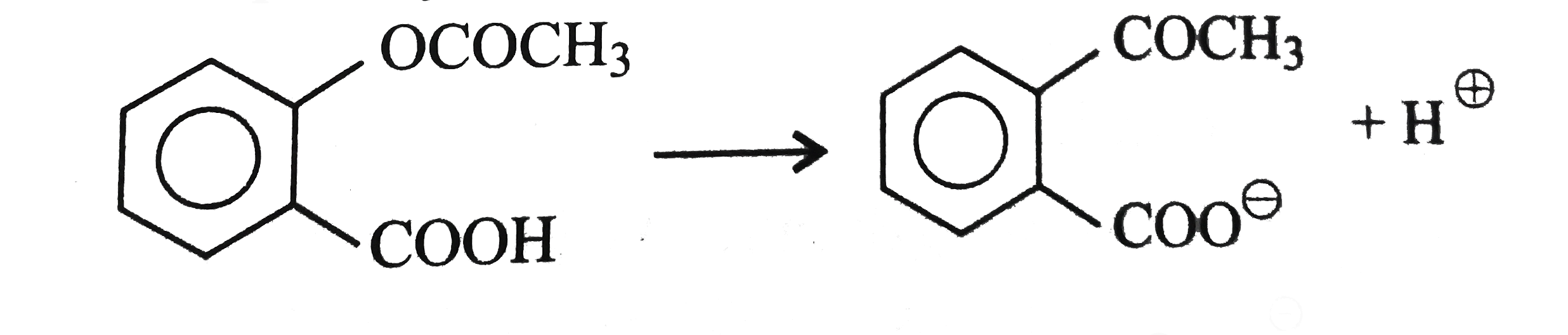
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
IONIC EQUILIBRIUM
CENGAGE CHEMISTRY|Exercise Exercises Assertion-Reasoning|36 VideosIONIC EQUILIBRIUM
CENGAGE CHEMISTRY|Exercise Exercises Integer|10 VideosIONIC EQUILIBRIUM
CENGAGE CHEMISTRY|Exercise Exercises Multiple Correct|33 VideosHYDROGEN, WATER AND HYDROGEN PEROXIDE
CENGAGE CHEMISTRY|Exercise Subjective Archive (Subjective)|3 VideosISOMERISM
CENGAGE CHEMISTRY|Exercise Assertion-Reasoning Type|1 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-IONIC EQUILIBRIUM-Exercises Single Correct
- A solution with pH = 12 is more acidic then one with a pH = 6 by a fac...
Text Solution
|
- A definite volume of an aqueous N//20 acetic acid (pK(a) = 4.74) is ti...
Text Solution
|
- The pK(a) of acteylsalicylic acid (aspirin) is 3.5. The pH of gastric ...
Text Solution
|
- Which of the following salt is basic?
Text Solution
|
- For the indicator 'Hin' the ratio (Ind^(Θ))//(HIn) is 7.0 at pH of 4.3...
Text Solution
|
- When 0.002mol of acid is added to 250 mL of a buffer solution, pH decr...
Text Solution
|
- pH of an aqueous solution of 0.6M NH(3) and 0.4M NH(4)CI is 9.4 (pK(b)...
Text Solution
|
- Which of the following salts undergoes anionic hydrolysis?
Text Solution
|
- A saturated solution of Ag(2)SO(4) is 2.5 xx 10^(-2)M. The value of it...
Text Solution
|
- Which one of the followinf is acid salt?
Text Solution
|
- Which one is not an acid salt?
Text Solution
|
- Which one of the following salts when dissolves in water hydrolyse?
Text Solution
|
- Which of the following salt undergoes hydrolysis?
Text Solution
|
- Out of the following the compound whose water solution has the highest...
Text Solution
|
- When equal volumes of the following solutions are mixed, precipitation...
Text Solution
|
- The gatric juice in our stomach contains enough HCI to make the hydrog...
Text Solution
|
- Of the given anions, the strongest Bronsted base is
Text Solution
|
- In decinormal solution, CH(3)COOH acid is ionised to the extent of 1.3...
Text Solution
|
- An aqueous solution of aluminium sulphate would show
Text Solution
|
- The aqueous solution of AICI(3) is acidic due to
Text Solution
|
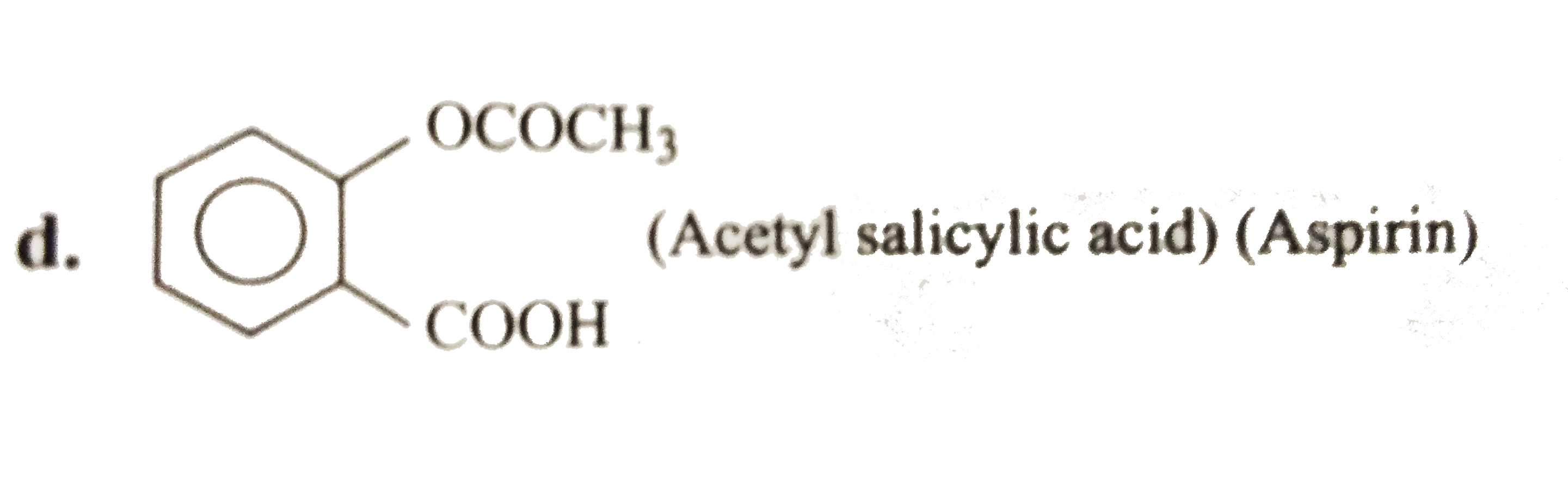 Aspirin is a weak acid and its ionisation is supressed due to common ion effect in acidic medium, i.e., in stomach. Therefore, aspirin is unionised is stomach whereas in small intestine its `pH` is basic. So the ionisation of aspirin increases, that is why it is completely ionised in small intenstine.
Aspirin is a weak acid and its ionisation is supressed due to common ion effect in acidic medium, i.e., in stomach. Therefore, aspirin is unionised is stomach whereas in small intestine its `pH` is basic. So the ionisation of aspirin increases, that is why it is completely ionised in small intenstine.