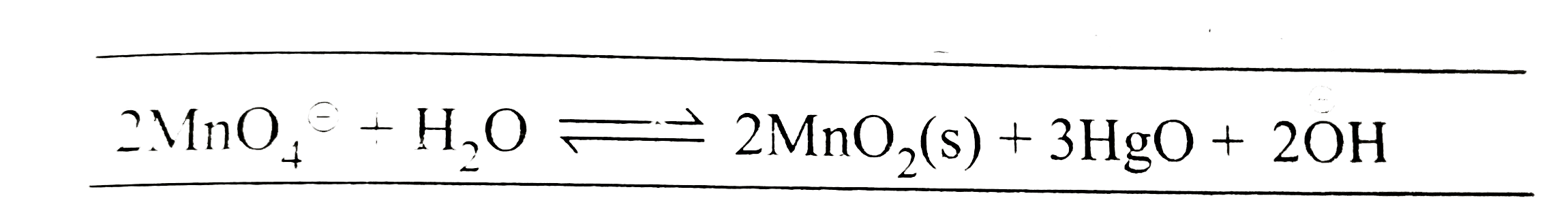Text Solution
Verified by Experts
Topper's Solved these Questions
ELECTROCHEMISTRY
CENGAGE CHEMISTRY|Exercise Solved Examples(Electrolysis And Electrolytic Cells)|12 VideosELECTROCHEMISTRY
CENGAGE CHEMISTRY|Exercise Ex 3.1 (Objective)|28 VideosELECTROCHEMISTRY
CENGAGE CHEMISTRY|Exercise Archieves Subjective|35 VideosD AND F BLOCK ELEMENTS
CENGAGE CHEMISTRY|Exercise Archives Subjective|29 VideosGENERAL PRINCIPLES AND PROCESS OF ISOLATION OF ELEMENTS
CENGAGE CHEMISTRY|Exercise Archives (Subjective)|14 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-ELECTROCHEMISTRY-Solved Examples (Electrochemical Cell)
- Given : NO(3)^(c-) rarrNO(2)( acidic medium ), " "E^(c-)=0.8V ...
Text Solution
|
- The standard potential of a cell using the reaction is 1.12. The heat...
Text Solution
|
- The standard potential of a cell using the reaction +3HgO(s)+2(overse...
Text Solution
|
- The EMF of the cell : Ag|AgCl,0.1 MKCl||0.1 M AgNO(3)|Ag is 0.45V. 0...
Text Solution
|
- Calculate the potential corresponding to the following cell. Given P...
Text Solution
|
- Estimate the standard reduction potential for the copper // copper sul...
Text Solution
|
- Knowing that K(sp) for AgCl is 1.0 xx 10^(-10), calculate E for a silv...
Text Solution
|
- Consider the following half reactions : PbO(2)(s)+4H^(o+)(aq)+SO(4)...
Text Solution
|
- The EMF of the cell Ag|0.1(N)AgNO(3)||1(N) KBr, AgBr(s)|Ag was found...
Text Solution
|
- For the cell Zn|ZnCl(2)(m)|AgCl,E is 1.24V at 25^(@)C and 1.260V at 35...
Text Solution
|
- A saturated calomel electrode is coupled through a salt bridge with a ...
Text Solution
|
- Two weak acid solutions HA(1) and HA(2) with the same concentration an...
Text Solution
|
- Find the solubility of AgCl in 0.1 M CaCl(2). E^(c-).(Ag^(o+)|Ag)=0.79...
Text Solution
|
- The EMF of the cell : Ag|Ag(2)CrO(4)(s),K(2)CrO(4)(0.1 M)||AgNO(3)(0...
Text Solution
|
- The EMF of a galvanic cell Pt|H(2)(1 atm)|HCl(1M)|Cl(2)(g)|Pt is 1.29V...
Text Solution
|
- Calculate the potential of silver electrode in a saturated solution of...
Text Solution
|
- A solution of Fe^(2+) is titrated potentiaometrically using Ce^(4+) so...
Text Solution
|
- Find the EMF of the cell at 25^(@)C. E^(c-).(red("quinhydrone elec...
Text Solution
|
- The EMF of the following cell is found to be -0.46V: If the stan...
Text Solution
|
- The EMF of the following cell is observed to be 0.118V at 25^(@)C: ...
Text Solution
|
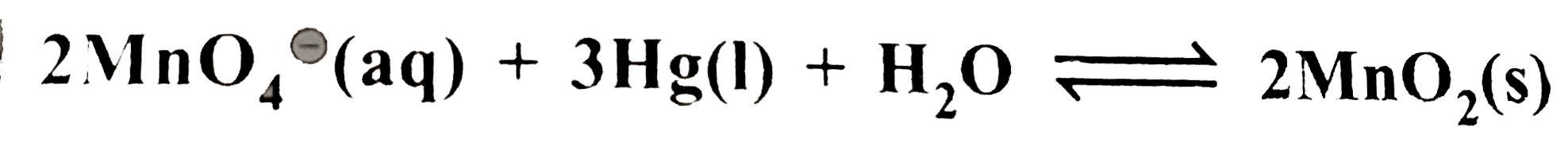 `+3HgO(s)+2(overset(c-)(O)H) (aq)` is `0.489V` at `25^(@)C`. What is the equilibrium constant of the reaction ?
`+3HgO(s)+2(overset(c-)(O)H) (aq)` is `0.489V` at `25^(@)C`. What is the equilibrium constant of the reaction ?