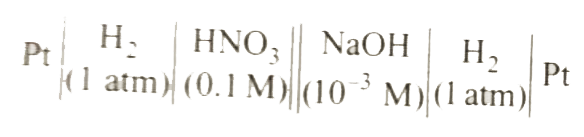A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ELECTROCHEMISTRY
CENGAGE CHEMISTRY|Exercise Exerciseassertion -Reasoning|25 VideosELECTROCHEMISTRY
CENGAGE CHEMISTRY|Exercise Exerciseinterger|8 VideosELECTROCHEMISTRY
CENGAGE CHEMISTRY|Exercise Exercisemultiple Correct Ansers|53 VideosD AND F BLOCK ELEMENTS
CENGAGE CHEMISTRY|Exercise Archives Subjective|29 VideosGENERAL PRINCIPLES AND PROCESS OF ISOLATION OF ELEMENTS
CENGAGE CHEMISTRY|Exercise Archives (Subjective)|14 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-ELECTROCHEMISTRY-Exercises Ingle Correct
- The variation equivalent conductance of stronge electrolyte with sqrt(...
Text Solution
|
- Given the standard potential of the following at 25^(@)C. MnO(2) ra...
Text Solution
|
- The potential of the following cell at 25^(@)C is
Text Solution
|
- Given the following cell at 25^(@)C What will be the potential of...
Text Solution
|
- What is the potential of the cell containing two hydrogen electrode as...
Text Solution
|
- Given electrode potentials asre Fe^(3+)+e^(-) rarr Fe^(2+)," "E^(...
Text Solution
|
- The following facts are availabel : 2A^(c-)+B(2) rarr 2B^(-)+A(2), ...
Text Solution
|
- The potential the cell at 25^(@)C is Given pK(b) of NH(4)OH=4.74
Text Solution
|
- The potential of the cell at 25^(@)C is Given pK(a) of CH(3)COOH...
Text Solution
|
- Which metal can deposit copper from copper sulphate solution ?
Text Solution
|
- On the basis of position in the electrolchemical series, the metal whi...
Text Solution
|
- A dilute aqueous solution of sodium fluoride is electrolyzed, the prod...
Text Solution
|
- Copper can be deposited from acidified copper sulphate and alkaline cu...
Text Solution
|
- Silver is removed electrolytically from 200mL of a 0.1N solution of Ag...
Text Solution
|
- Chromium plating can involve the electrolysis of an electrolyte of an ...
Text Solution
|
- Which of the following does not evolve oxygen at anode when the elect...
Text Solution
|
- Calculate the potential of the following cell : Pt|underset((2.0M)(...
Text Solution
|
- The electricity conductivity of a solution serves as a means of determ...
Text Solution
|
- A constant current was passed through a solution of AuCl(4)^(c-) ion b...
Text Solution
|
- E^(c-) for FeY^(c-)+e^(c-) rarr FeY^(2-)
Text Solution
|