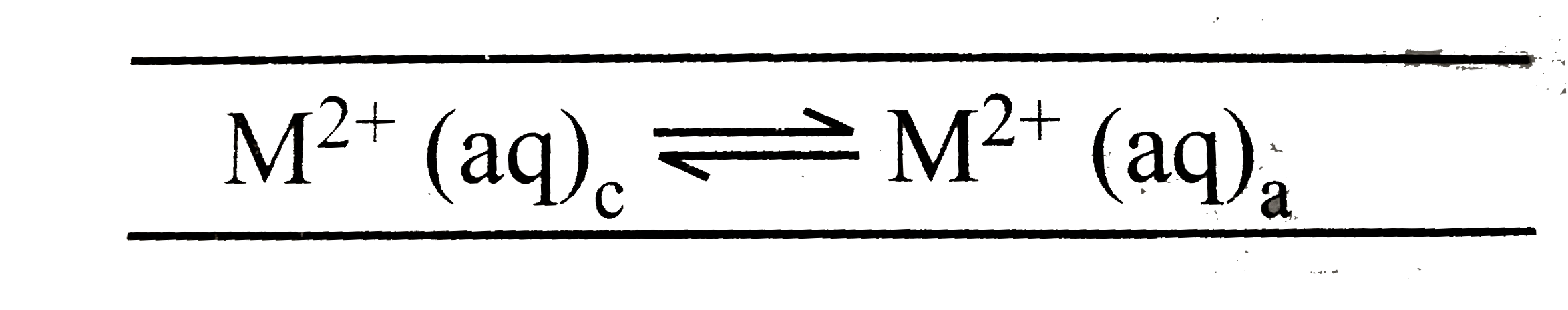
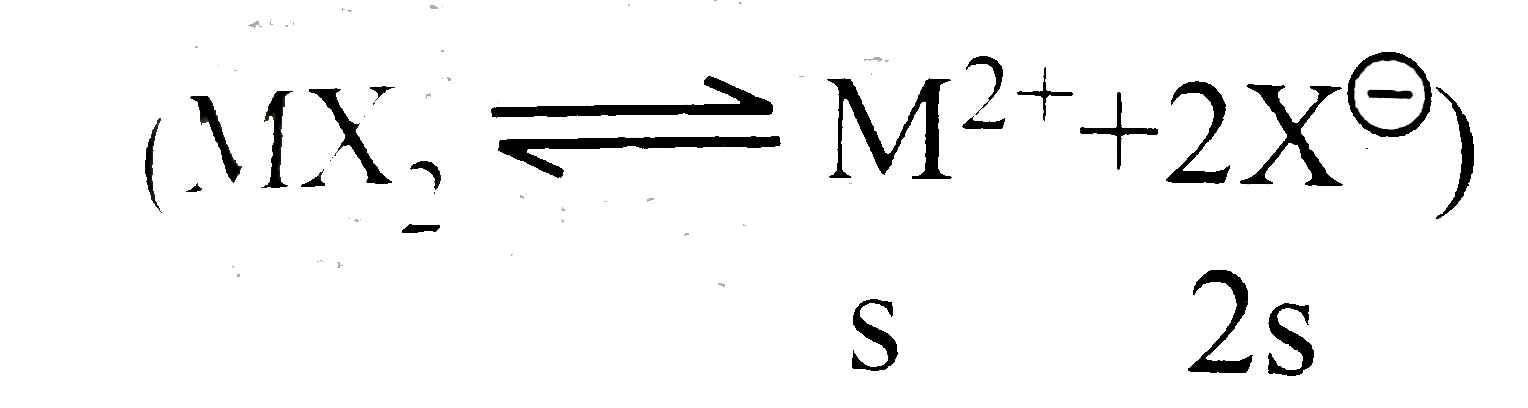A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ELECTROCHEMISTRY
CENGAGE CHEMISTRY|Exercise Archieves Multiple Correct Ansers|2 VideosELECTROCHEMISTRY
CENGAGE CHEMISTRY|Exercise Archieves Single Correct|25 VideosELECTROCHEMISTRY
CENGAGE CHEMISTRY|Exercise Exercisetrue / False|40 VideosD AND F BLOCK ELEMENTS
CENGAGE CHEMISTRY|Exercise Archives Subjective|29 VideosGENERAL PRINCIPLES AND PROCESS OF ISOLATION OF ELEMENTS
CENGAGE CHEMISTRY|Exercise Archives (Subjective)|14 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-ELECTROCHEMISTRY-Archieves (Linked Comprehension )
- Tollen reagent is used for the detection of aldehydes. When a solution...
Text Solution
|
- Tollen reagent is used for the detection of aldehydes. When a solution...
Text Solution
|
- Tollen reagent is used for the detection of aldehydes. When a solution...
Text Solution
|
- Chemical reactions involve interation of atoms and molecules. A large ...
Text Solution
|
- Chemical reactions involve interation of atoms and molecules. A large ...
Text Solution
|
- Chemical reactions involve interation of atoms and molecules. A large ...
Text Solution
|
- Redox reactions play a pivotal role in chemistry and biology. The valu...
Text Solution
|
- Redox reactions play a pivotal role in chemistry and biology. The valu...
Text Solution
|
- Redox reactions play a pivotal role in chemistry and biology. The valu...
Text Solution
|
- The concentration of potassium ions inside a biological cell is at lea...
Text Solution
|
- The concentration of potassium ions inside a biological cell is at lea...
Text Solution
|
- The electrochemical cell shown below is a concentration cell. M|M^(2+)...
Text Solution
|
- Consider Zn+Cu^(2+)rarrZn^(2+)+Cu If the standard emf is E("cell")=2...
Text Solution
|