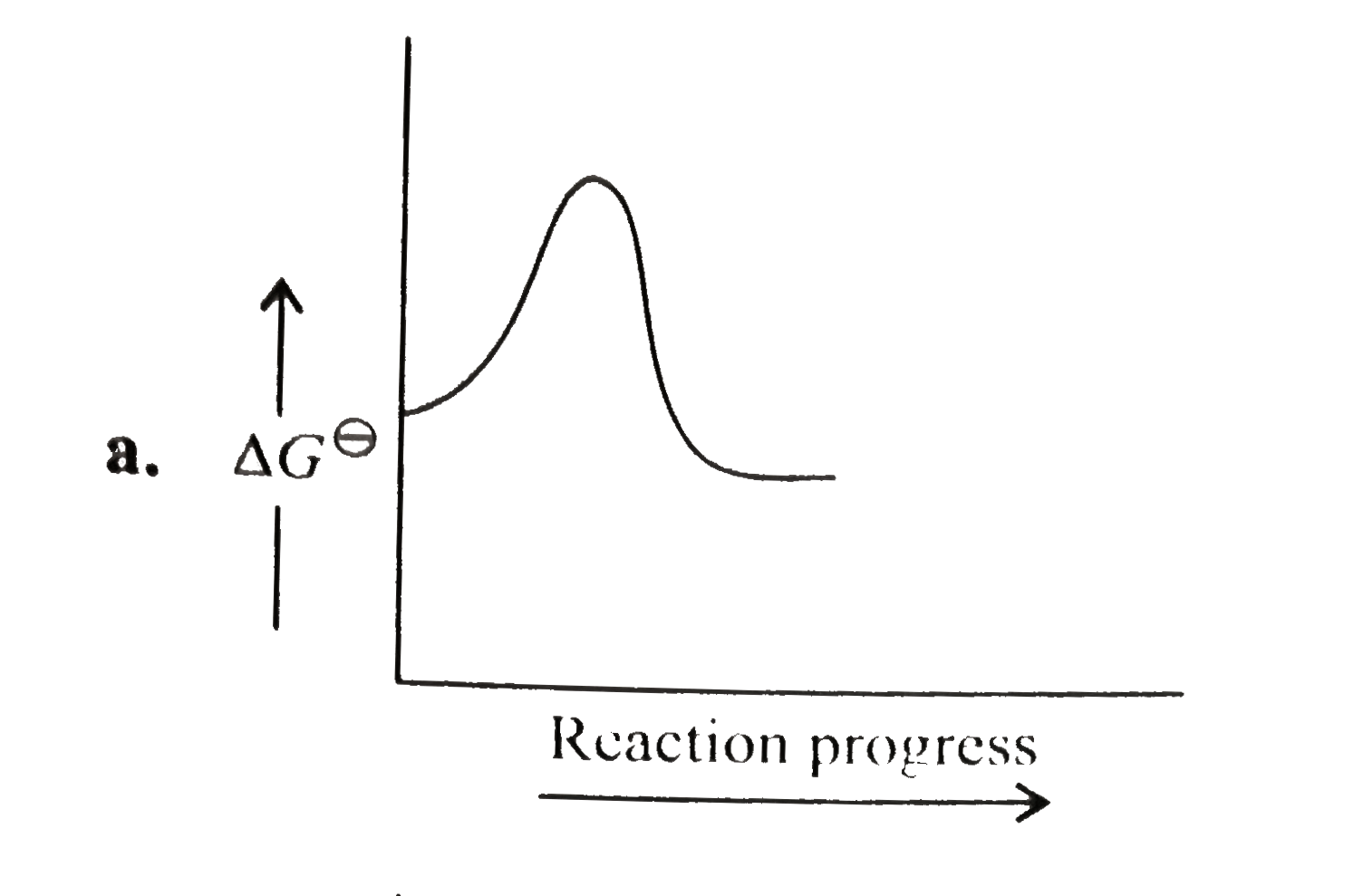
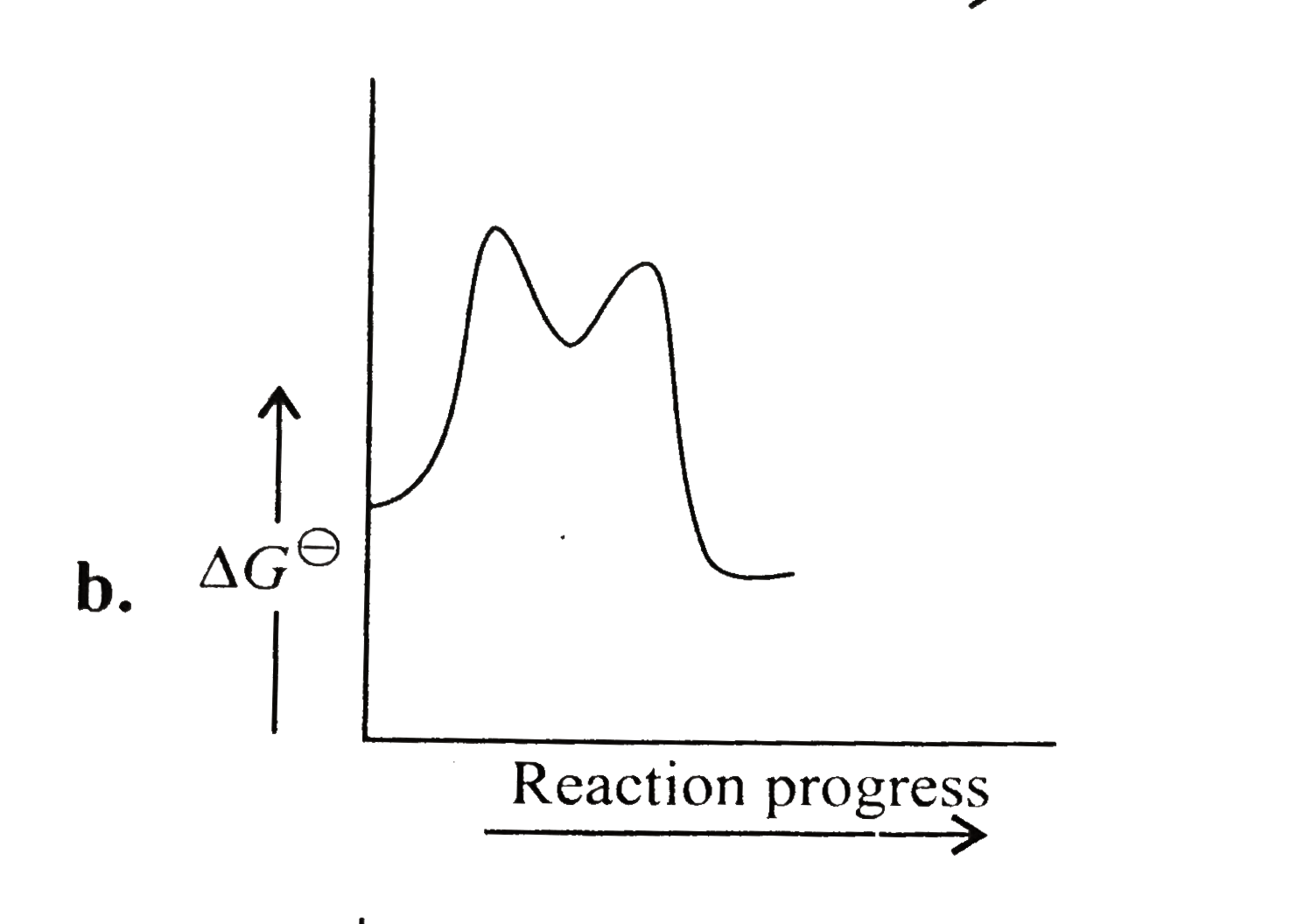
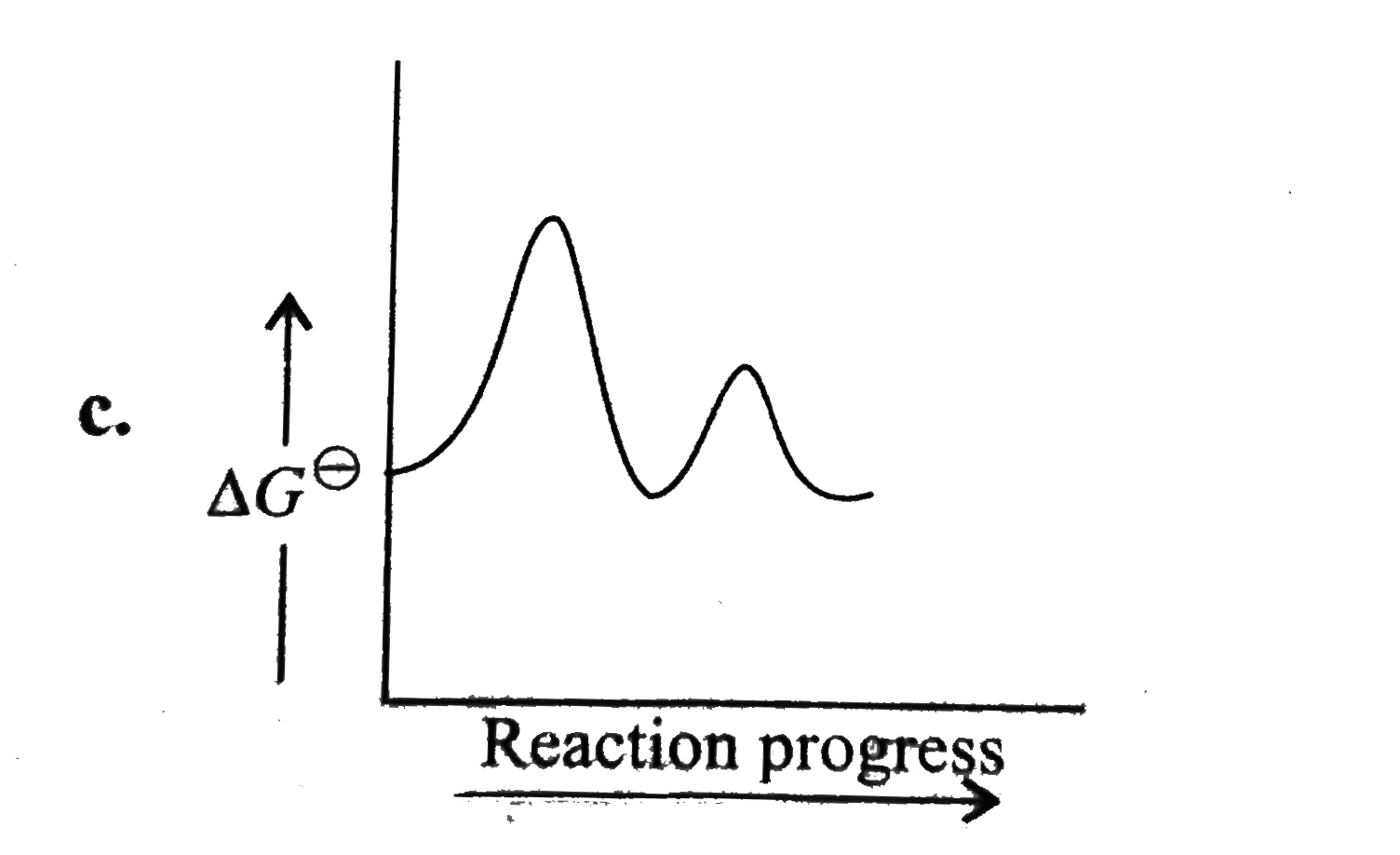
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL KINETICS
CENGAGE CHEMISTRY|Exercise Ex 4.3 More Than One Correct|5 VideosCHEMICAL KINETICS
CENGAGE CHEMISTRY|Exercise Ex4.4 Objective|10 VideosCHEMICAL KINETICS
CENGAGE CHEMISTRY|Exercise Ex 4.2 (Objective)|10 VideosCARBOXYLIC ACIDS AND THEIR DERIVATIVES
CENGAGE CHEMISTRY|Exercise Exercises Archives (Analytical And Descriptive)|34 VideosCOORDINATION COMPOUNDS
CENGAGE CHEMISTRY|Exercise Archives Subjective|18 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-CHEMICAL KINETICS-Ex 4.3 (Objective)
- Aqueous AB(2) decomposes according to the first order reaction: AB(2...
Text Solution
|
- A plot of logarithm of rate vs logarithm of concentration of the react...
Text Solution
|
- The rate of radioactive decay of a sample are 3 xx 10^(8) dps and 3 xx...
Text Solution
|
- Consider the following parallel reactions being given by A(t(1//2) = 1...
Text Solution
|
- The rate constant for forward reaction A(g) hArr 2B(g) is 1.5 xx 10^(...
Text Solution
|
- The rate law for a reaction between A and B is given by rate = k[A]^(n...
Text Solution
|
- During the study of kinetics of chemical or nuclear reaction, t(1//3) ...
Text Solution
|
- Gadolinium-153, which is used to detect osteoporoiss, has a life of 24...
Text Solution
|
- The rate constant for the hydrolyiss of ethyl acetate in the presence ...
Text Solution
|
- For the reaction, R-X overset(Slow)rarrR^(o+)+X^(o+), R^(o+) + over...
Text Solution
|
- The rate constant for zero order reaction is
Text Solution
|
- Gaseous cyclobutane isomerizes to butadiene in a first order process w...
Text Solution
|
- The isomerization of cyclopropane to form propane is a first order rea...
Text Solution
|
- The converison of vinyl allyl ether to pent-4-enol follows first order...
Text Solution
|
- Calculate the half life of the first-order reaction: C(2)H(4)O(g) ra...
Text Solution
|