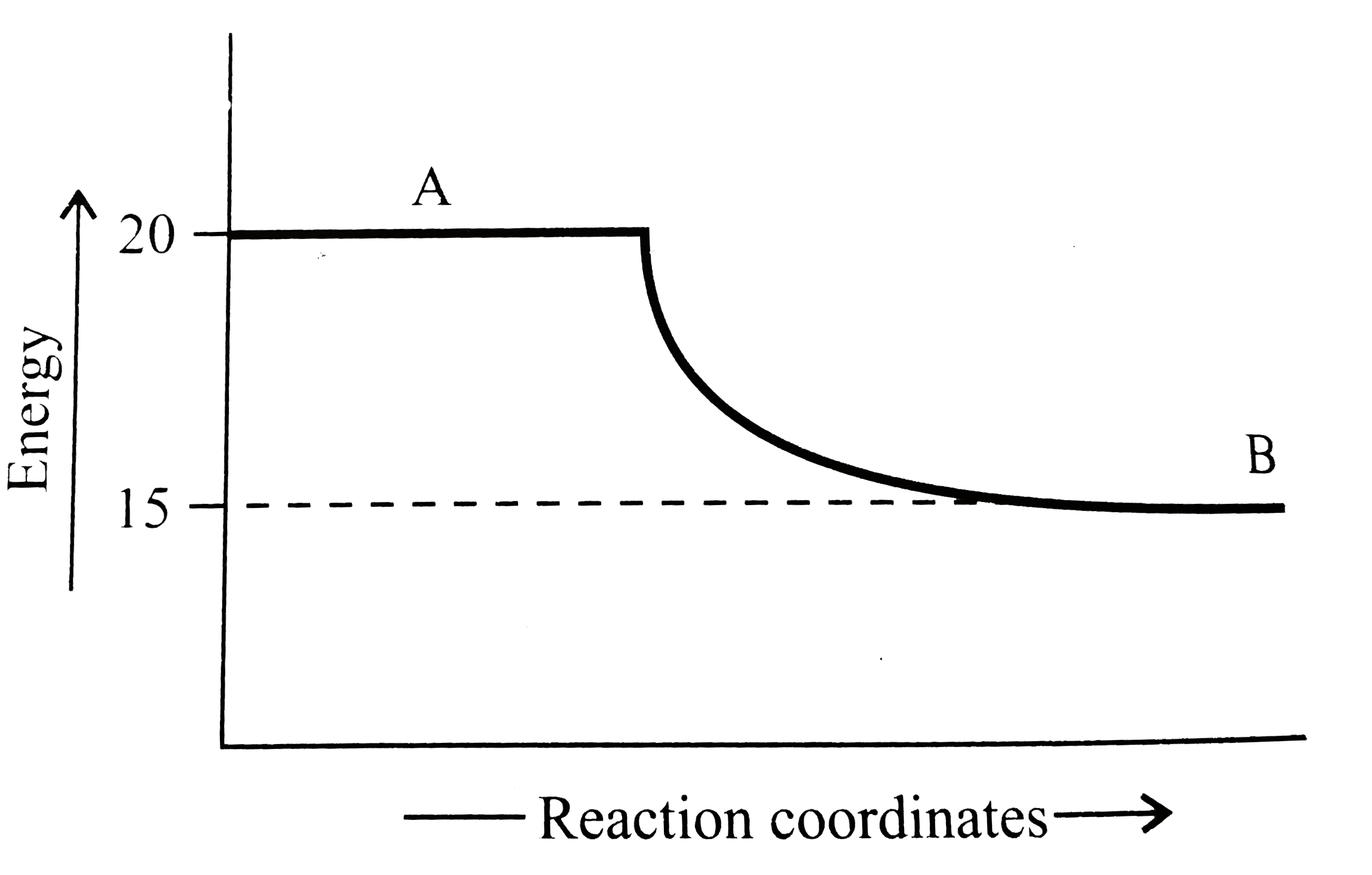
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL KINETICS
CENGAGE CHEMISTRY|Exercise Exercises Assertion-Reasoning|22 VideosCHEMICAL KINETICS
CENGAGE CHEMISTRY|Exercise Exercises Integer|15 VideosCHEMICAL KINETICS
CENGAGE CHEMISTRY|Exercise Exercises Multiple Correct|34 VideosCARBOXYLIC ACIDS AND THEIR DERIVATIVES
CENGAGE CHEMISTRY|Exercise Exercises Archives (Analytical And Descriptive)|34 VideosCOORDINATION COMPOUNDS
CENGAGE CHEMISTRY|Exercise Archives Subjective|18 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-CHEMICAL KINETICS-Exercises Single Correct
- The free energy change the due to a reaction is zero when
Text Solution
|
- What is Delta H for the reaction A + B rarr C where the mechanism invo...
Text Solution
|
- What can you say about the existence of A if the potential energy dia...
Text Solution
|
- In a multistep reaction such as A + B rarr Q rarr C. The potential en...
Text Solution
|
- In which statement is true ?
Text Solution
|
- Given the following two mechanisms, one with catalyst and the other wi...
Text Solution
|
- The mechanism for the overall reaction is A(2) + B rarr C A(2) r...
Text Solution
|
- Which of the following statement is correct
Text Solution
|
- For a chemical reaction 2X + Y rarrZ, the rate of appearance of Z is 0...
Text Solution
|
- A chemical reaction occurs as a result of colliisons between reacting ...
Text Solution
|
- For profucing effective colliisons, the colliding molecules must have
Text Solution
|
- A following mechanism has been proposed for a reaction: 2A+Brarr D+E...
Text Solution
|
- For a chemical reaction A rarr B, it is found that the rate of reacti...
Text Solution
|
- For a hypothetical reaction: A+B rarr Products, the rate law is r = k[...
Text Solution
|
- A hypothetical reaction A(2) + B(2) rarr 2AB follows the mechanism as ...
Text Solution
|
- The slowest step of a particular reaction is found to be 1//2X(2) + Y(...
Text Solution
|
- For hypothetical chemical reaction A rarr I, it is found that the reac...
Text Solution
|
- Calculate the overall order of a reaction which has the rate expresiso...
Text Solution
|
- The rate of reaction increases by the increase of temperature because
Text Solution
|
- Which of the following explains the increase of the reaction rate by c...
Text Solution
|