a.(i)`gt` (ii) `gt` (iv)`gt` (iii) `(-OH gt -COOH gt-CH(OH)CH_(3) gt -CH_(2)OH)`
b. `(iii) gt (i) gt (iv) (-NO_(2) gt -NH_(2) gt -C-=N`
c. `(ii) gt (i)` ,
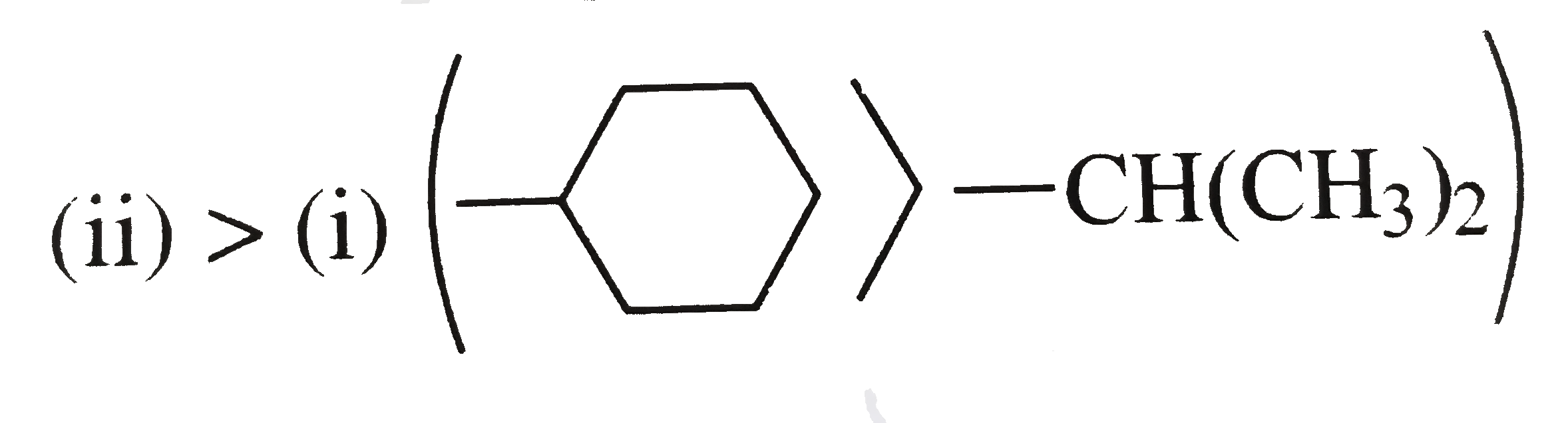
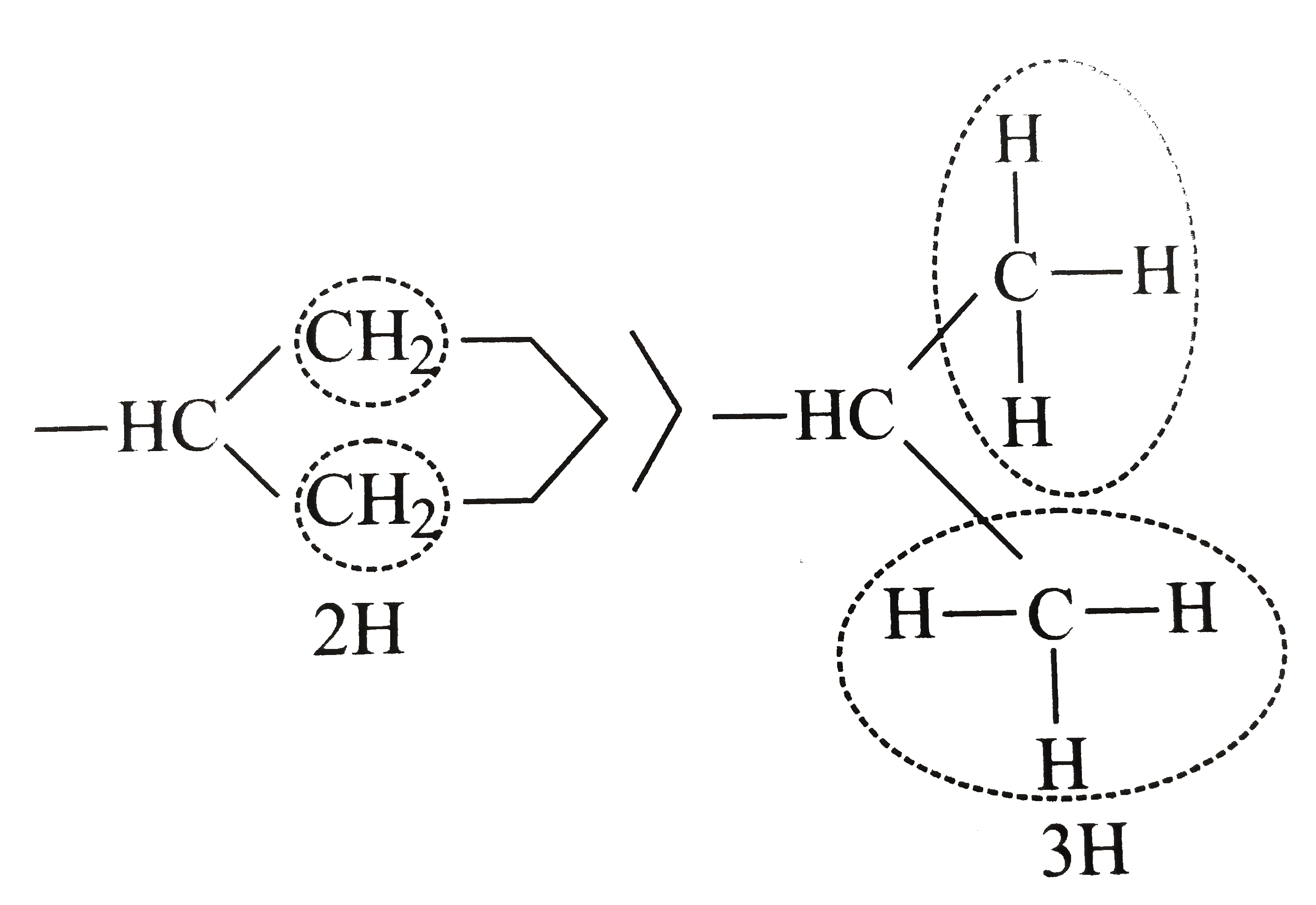
In cyclohexy, `(-CH)` is joined to two `(CH_(2))` groups and is further joined to another ring `C` atom.
In (i), `(-CH)` is joined to two `(CH_(3))` groups each having three `H` atoms.
d. (ii) `gt` (i)
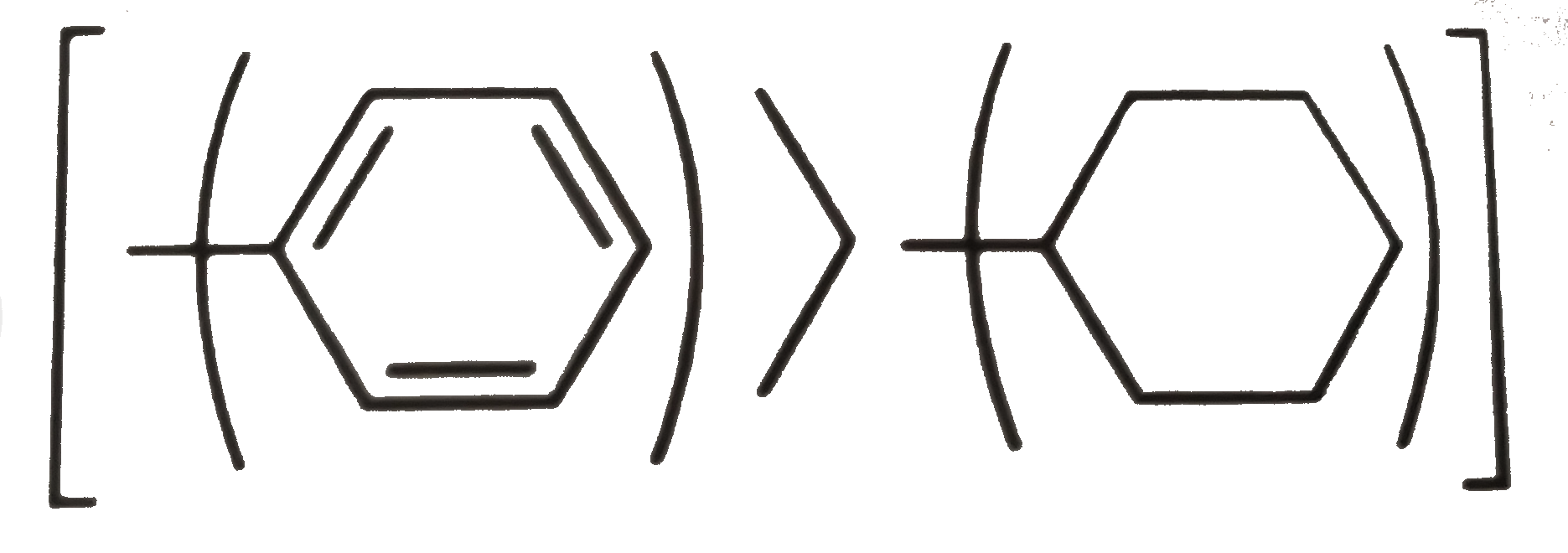
Phenyl `C` is doubly bonded and counted as attached to three `C` atoms.
e. `(i) gt (ii) ["Phenyl" gt -C(CH_(3))_(3)]`