a. `I gt II gt III`
(I).
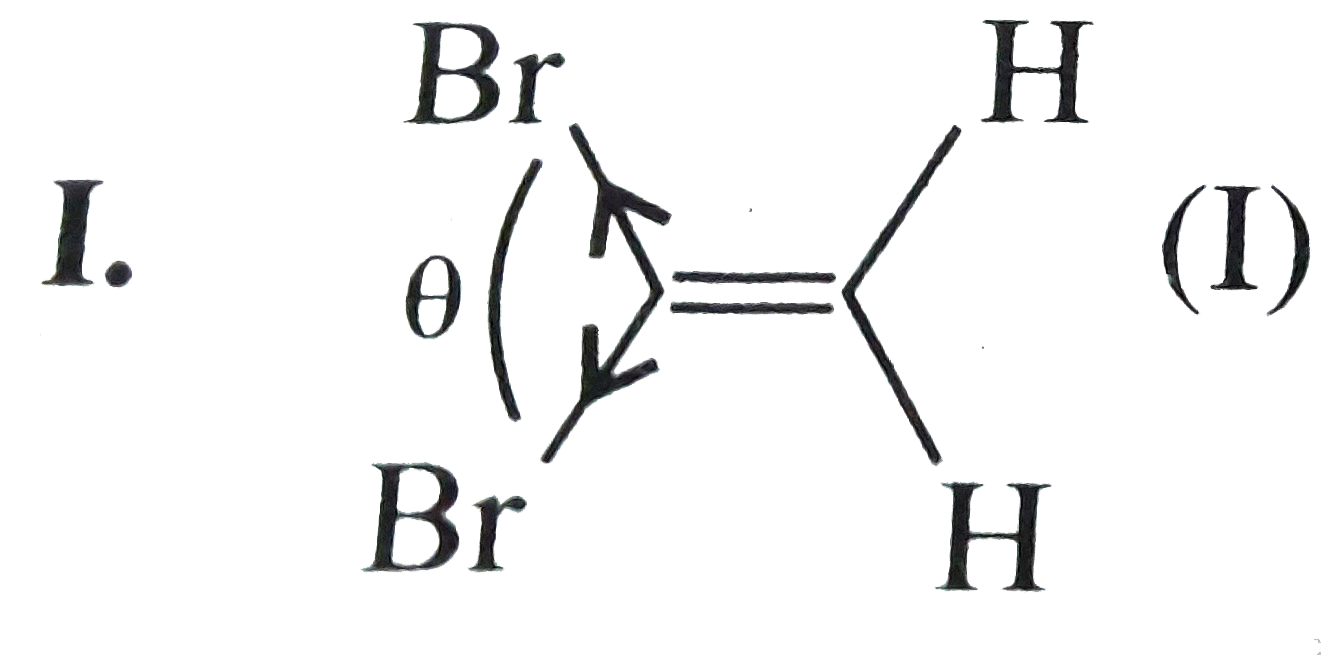
, (I) has a smaller angle of separation between two `Br` atoms than in (II) and (III) resulting in more net dipole moment `(mu)`.
(II).
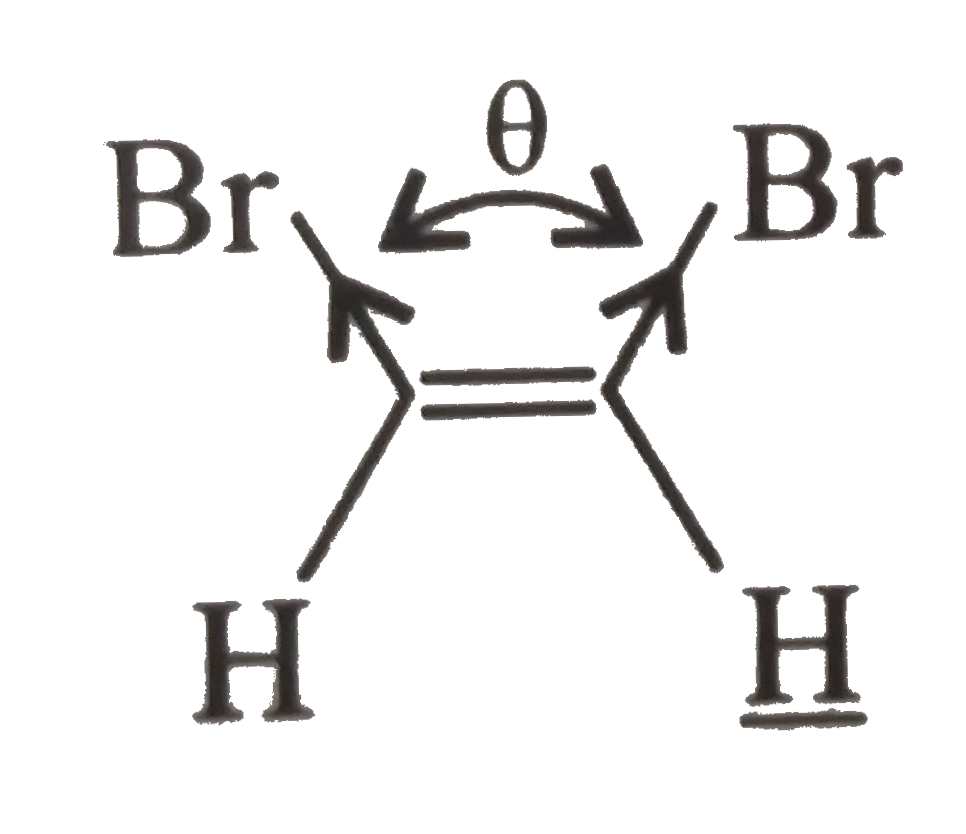
, (II) has a large angle of separation between two `Br`. atoms therefore, net `(mu)` is less than (I).
(III)
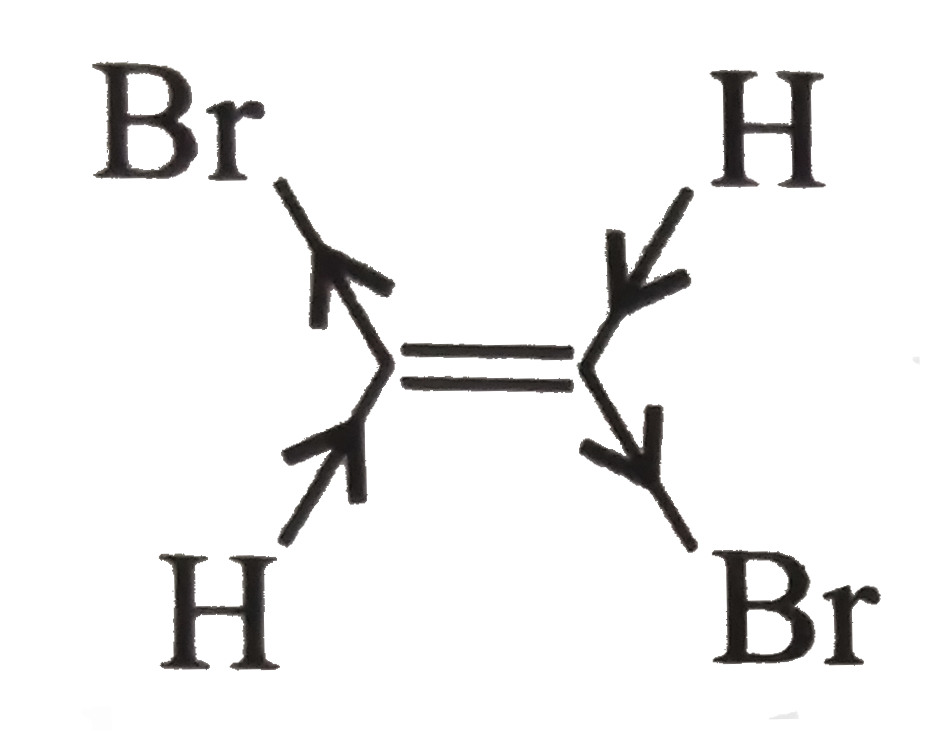
, In (III), two `Br` atoms and two `H` atoms have equal and opposite bond moments, therefore, net `mu` is zero.
b.`I gt II`.
(I).
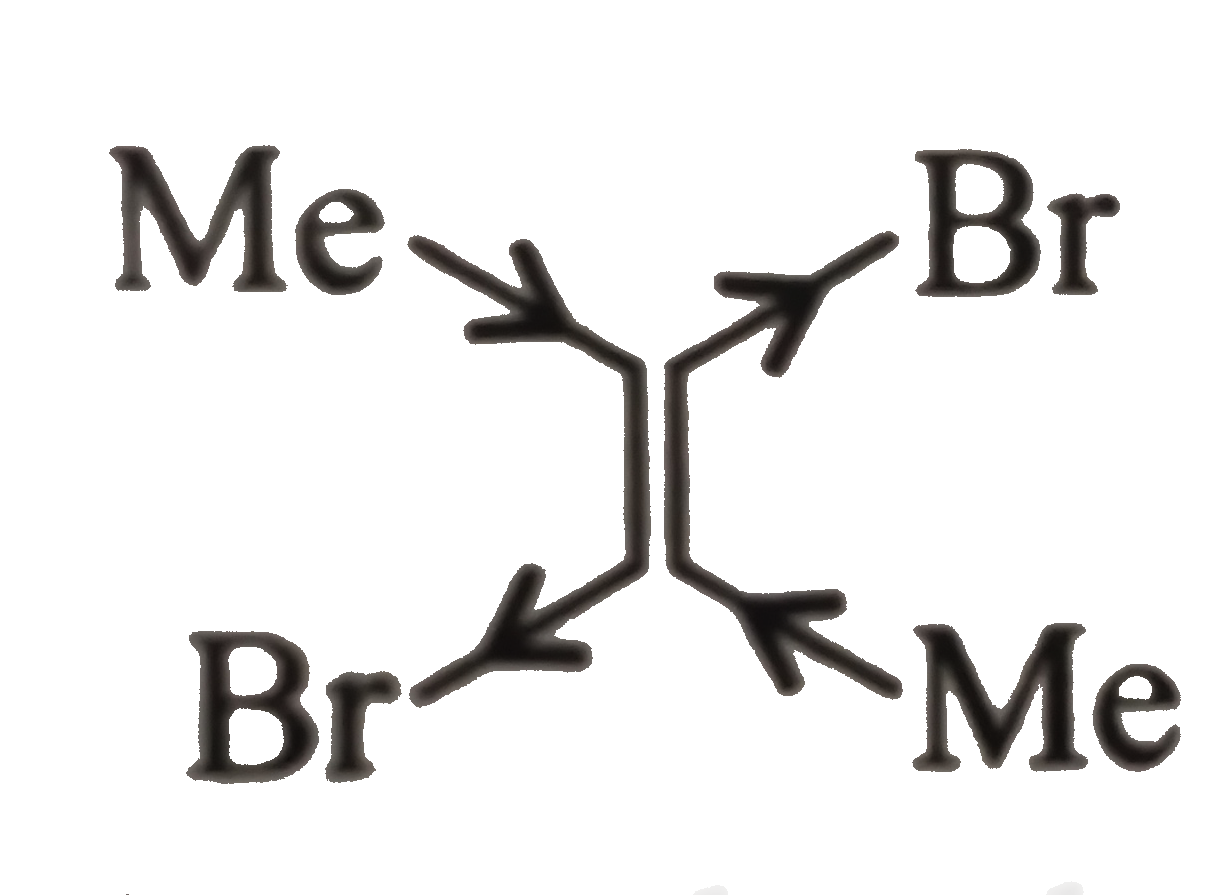
, Due to `vec(e )`- withdrawing `(-I)` effect of two `Br` atoms and `vec(e )`- donating `(+I)` effect of two `Me` atoms in the same direction, net resultant dipole moment is more.
(II).
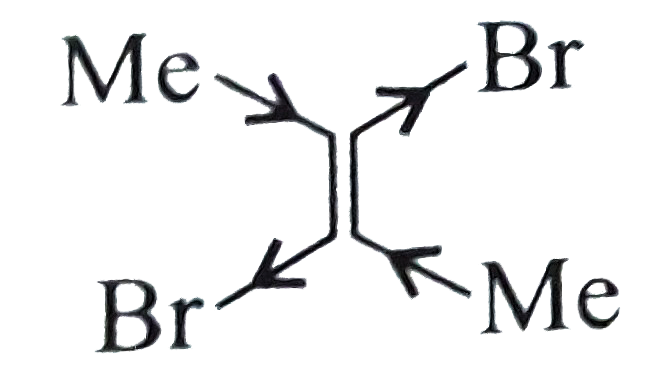
, Due to `vec(e )`-withdrawing `(-I)` effect of two `Br` atoms and `vec(e )` donating `(+I)` effect of two `Me` atoms, there is equal and opposite bond moment. Hence net resultant is zero.