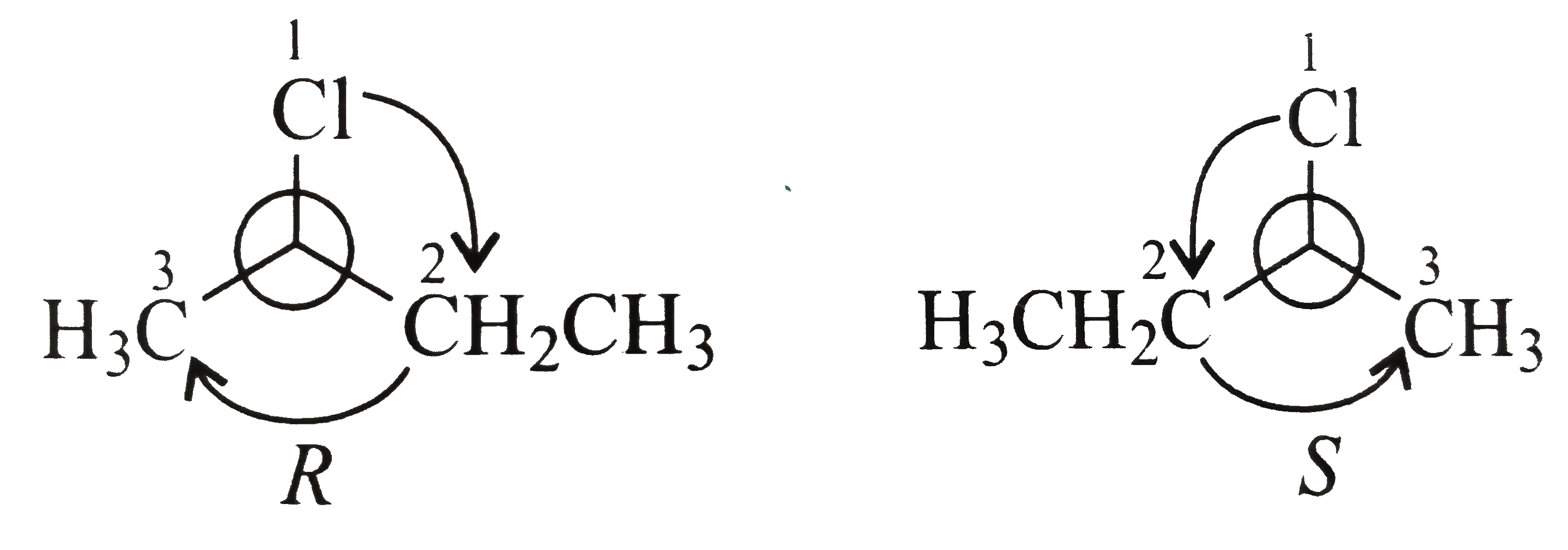Identification of the centre of chirality: Carbon-`2` bears four different substituents and hence it is the centre of chirality. (Carbon `1` and `4` bearing three hydrogen atoms each and Carbon-`3` bearing two `H` atoms cannot be chiral centres.)
ii. Deciding the order of priority for the four substituents using `CIP` rules.
`{:("Substituent:",CI,-CH_(2)CH_(3),-CH_(3),H),("Priority",1,2,3,4):}`
iii. Oreinting the molecule and configuration designation. `H` has the lowest priority and is to be kept farthest from the sight of the viewer and the molecule is to be looked down through the `C-H` bond and configuration assigned is based on the direction of the remaining three groups as shown in the following diagrams