a. Priority order, `-Br gt -C_(2)H_(5) gt -CH_(3) gt -H`.
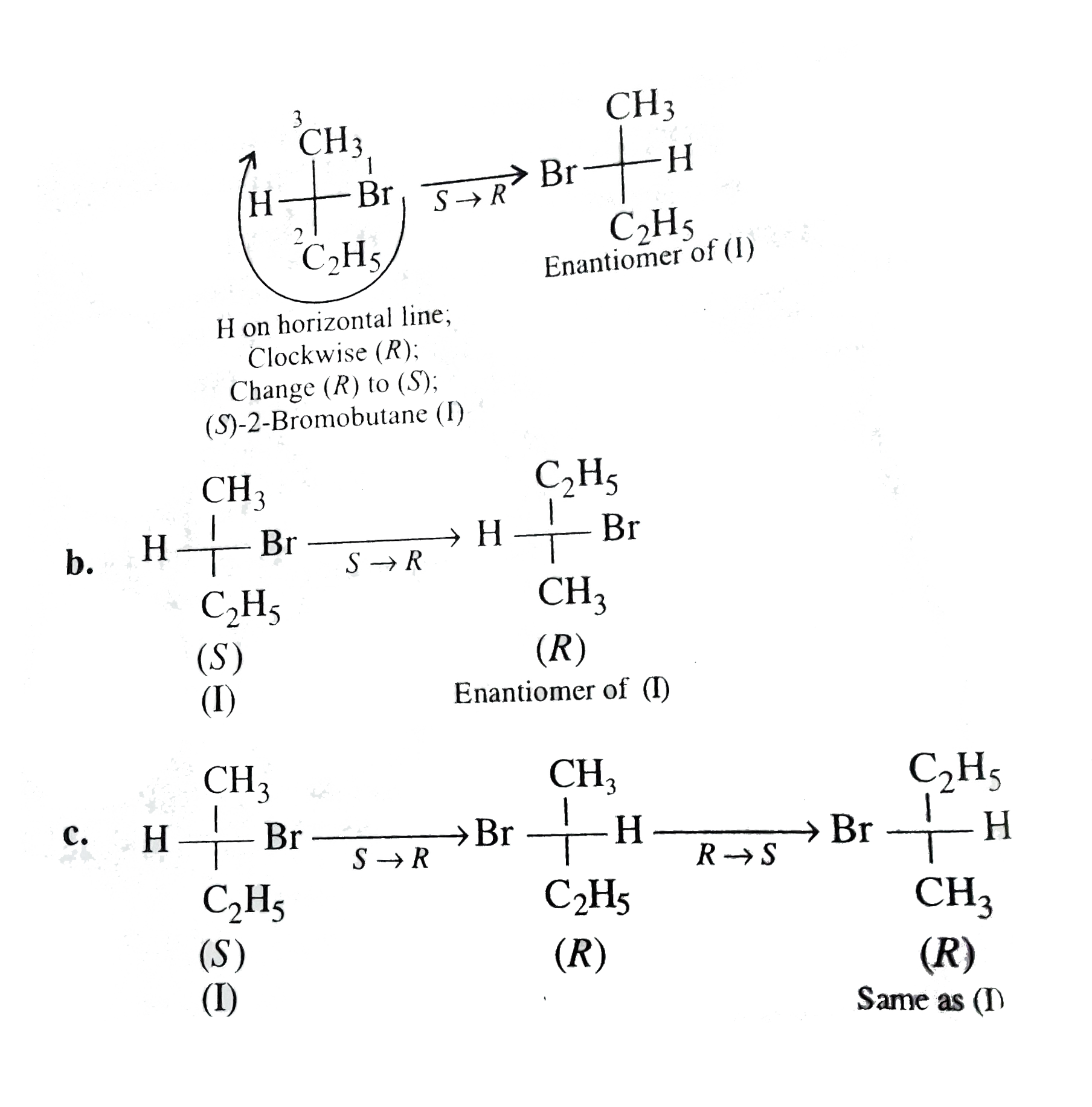
Thus, there is no change in the configuration when both switches (a) and (b) are made.
d.
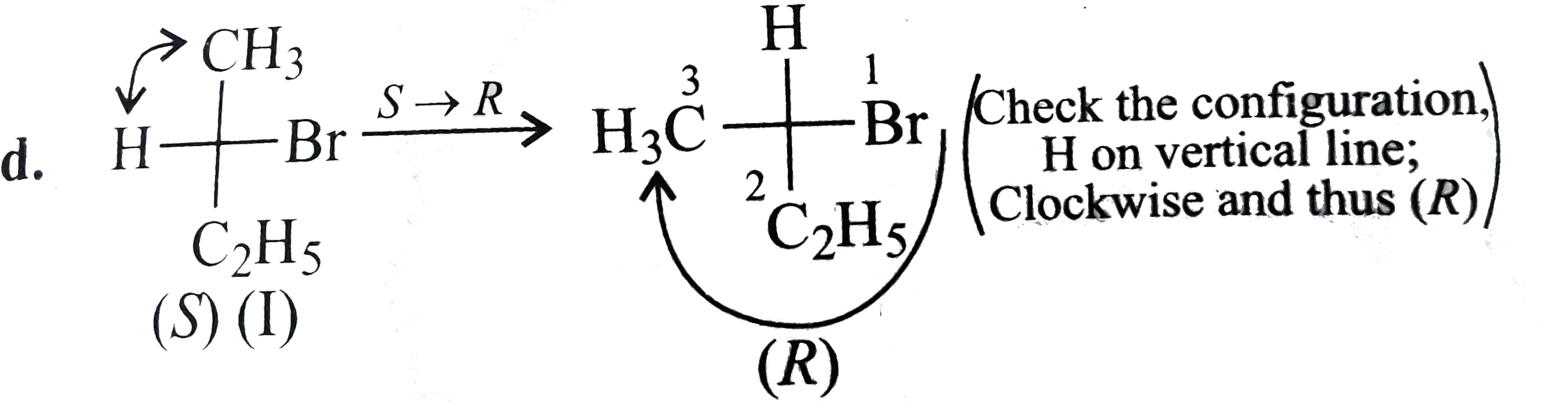
e. This type of operation is not allowed in Fischer projection beacuse any flipping out of the plane of paper (side to side or top to bottom) would change the ligands formely projecting behind the plane of paper to projecting towards the observer.
Those ligands formely pointing towards the observer would change to behind the paper.
f. This type of operation is also not allowed in Fischer projection. For example,
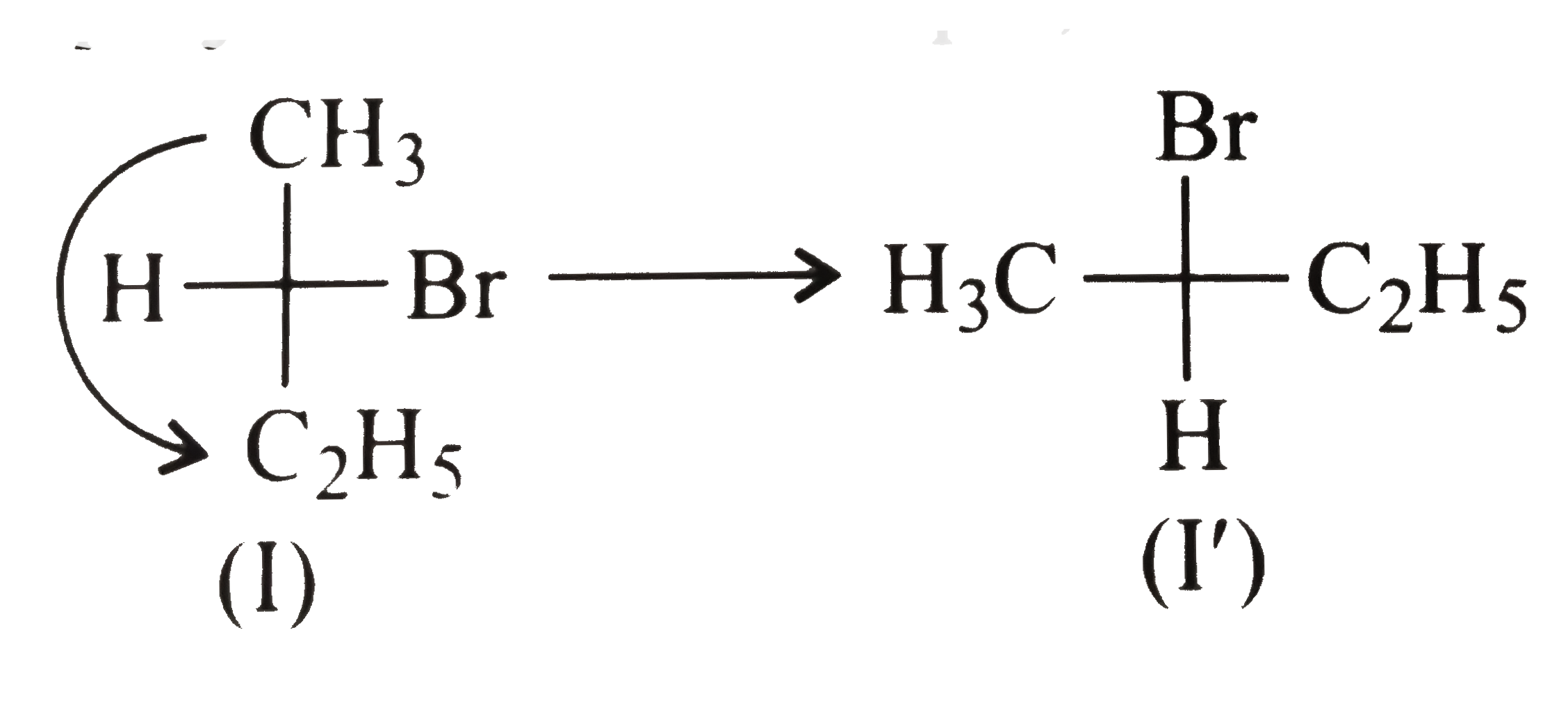
In (I) `(H)`, and `Br` project behind the plane, but a `90^(@)` rotation on the plane does not change the projection of bonds towards or away from the observer.