Text Solution
Verified by Experts
Topper's Solved these Questions
ISOMERISM
CENGAGE CHEMISTRY|Exercise (Linked Comprehension Type)Exercise|14 VideosISOMERISM
CENGAGE CHEMISTRY|Exercise Multiple choice questions (Exercise)|38 VideosISOMERISM
CENGAGE CHEMISTRY|Exercise Example|15 VideosIONIC EQUILIBRIUM
CENGAGE CHEMISTRY|Exercise Archives Subjective|28 VideosNCERT BASED EXERCISE
CENGAGE CHEMISTRY|Exercise Chemical Equilibrium|72 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-ISOMERISM-Exercise
- 0.90 gm of an organic compound C(4)H(10)O(2) (A) when treated with so...
Text Solution
|
- Write dwon the structrues of close homologues of heptane having one qu...
Text Solution
|
- An alkance (A) C(5)H(12) on chlorination at 300^(@)C gives a mixtrue o...
Text Solution
|
- Which alkane, having a molecular weight of 86, will form only two mono...
Text Solution
|
- A hydrocarbon (A) was found to have vapour density 36. If forms only a...
Text Solution
|
- Write the appropriate structural formule for: a. A cyclic molecule t...
Text Solution
|
- There are four dimethylcyclopropane isomers. a. Write the three-dime...
Text Solution
|
- Which of the follwing objects listed below possess a plane of symmetry...
Text Solution
|
- Assign (R ) or (S) designations to each of the following compounds a...
Text Solution
|
- Consider the following pair of structrue and tell whether they represe...
Text Solution
|
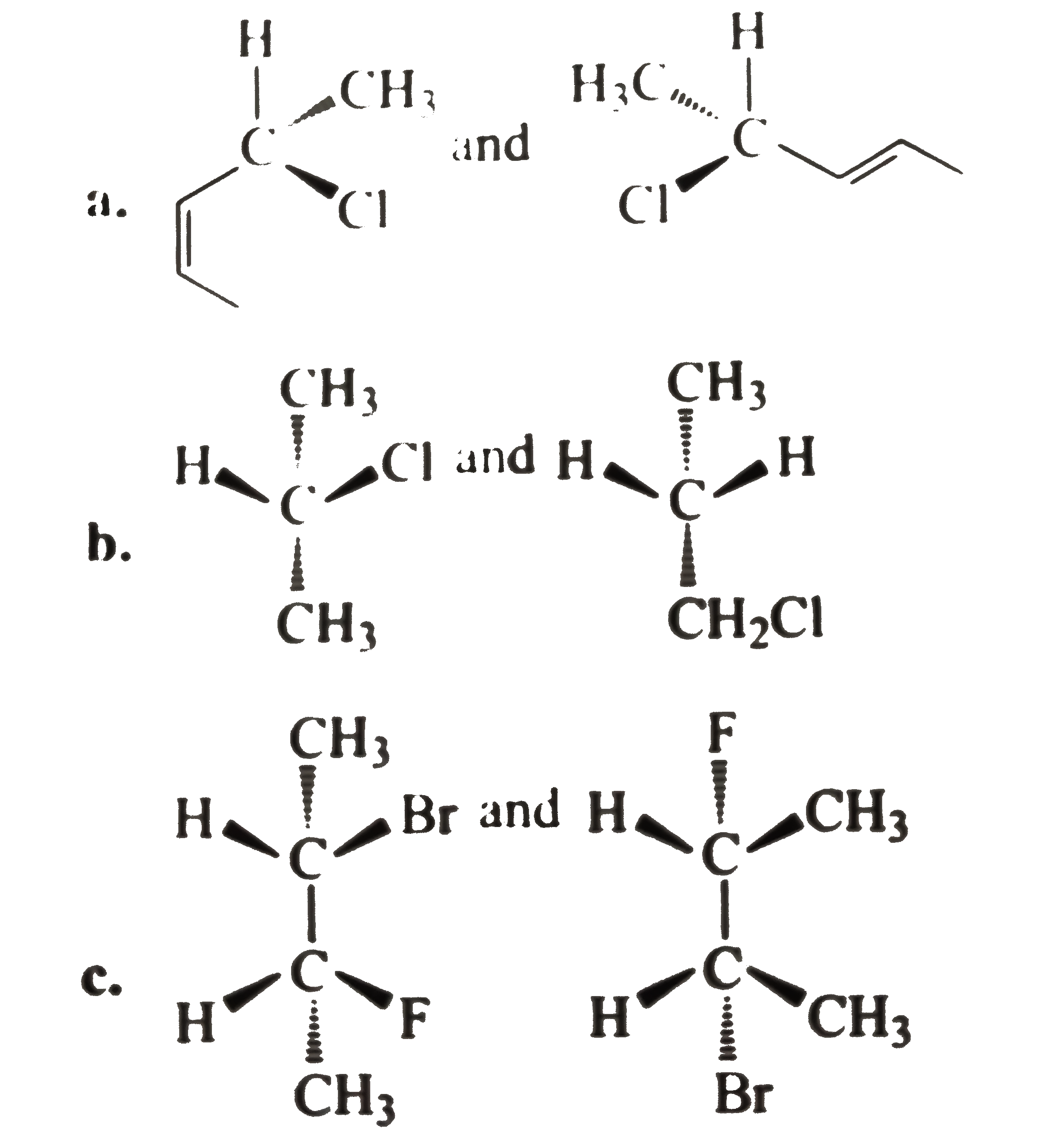
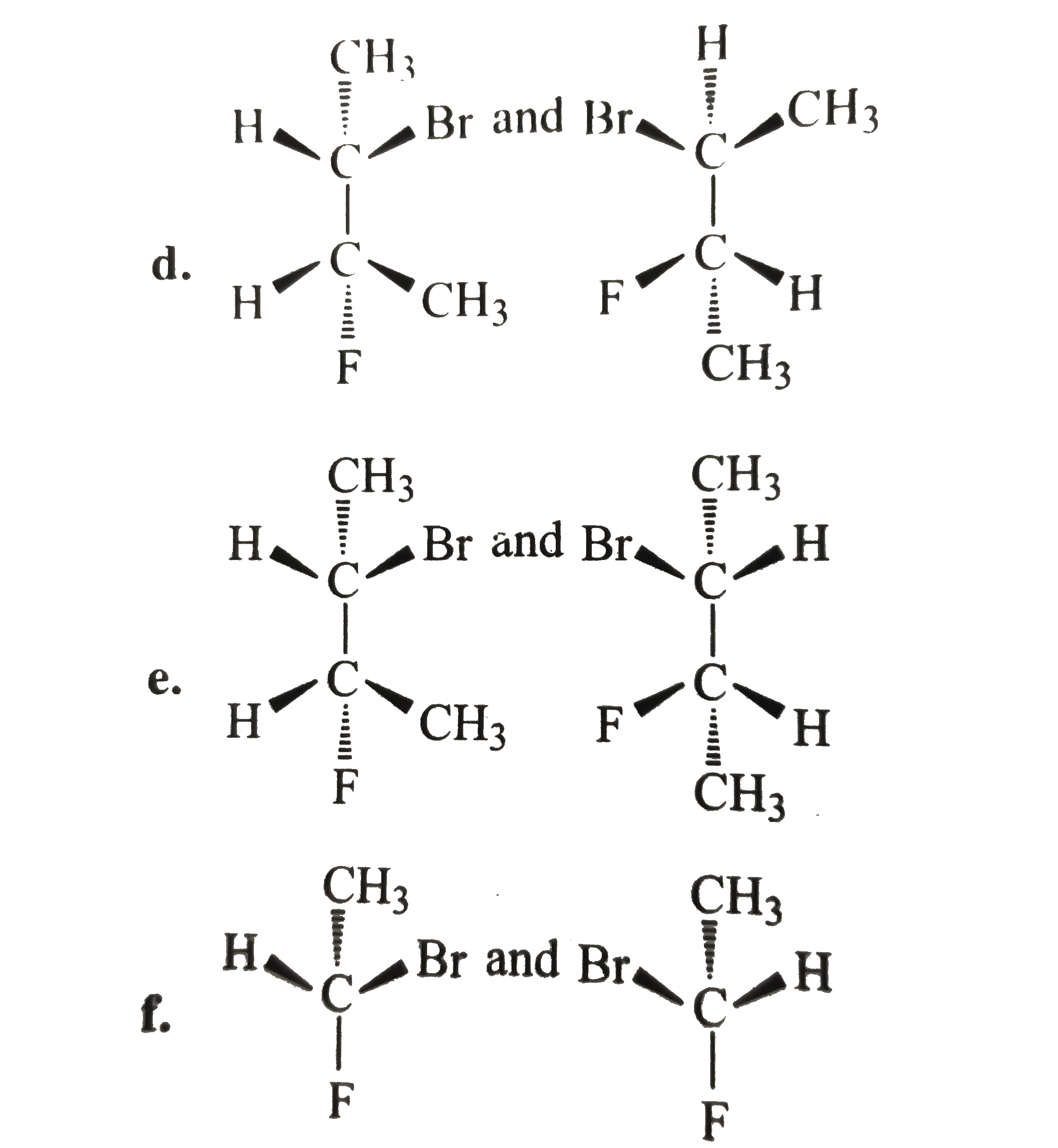
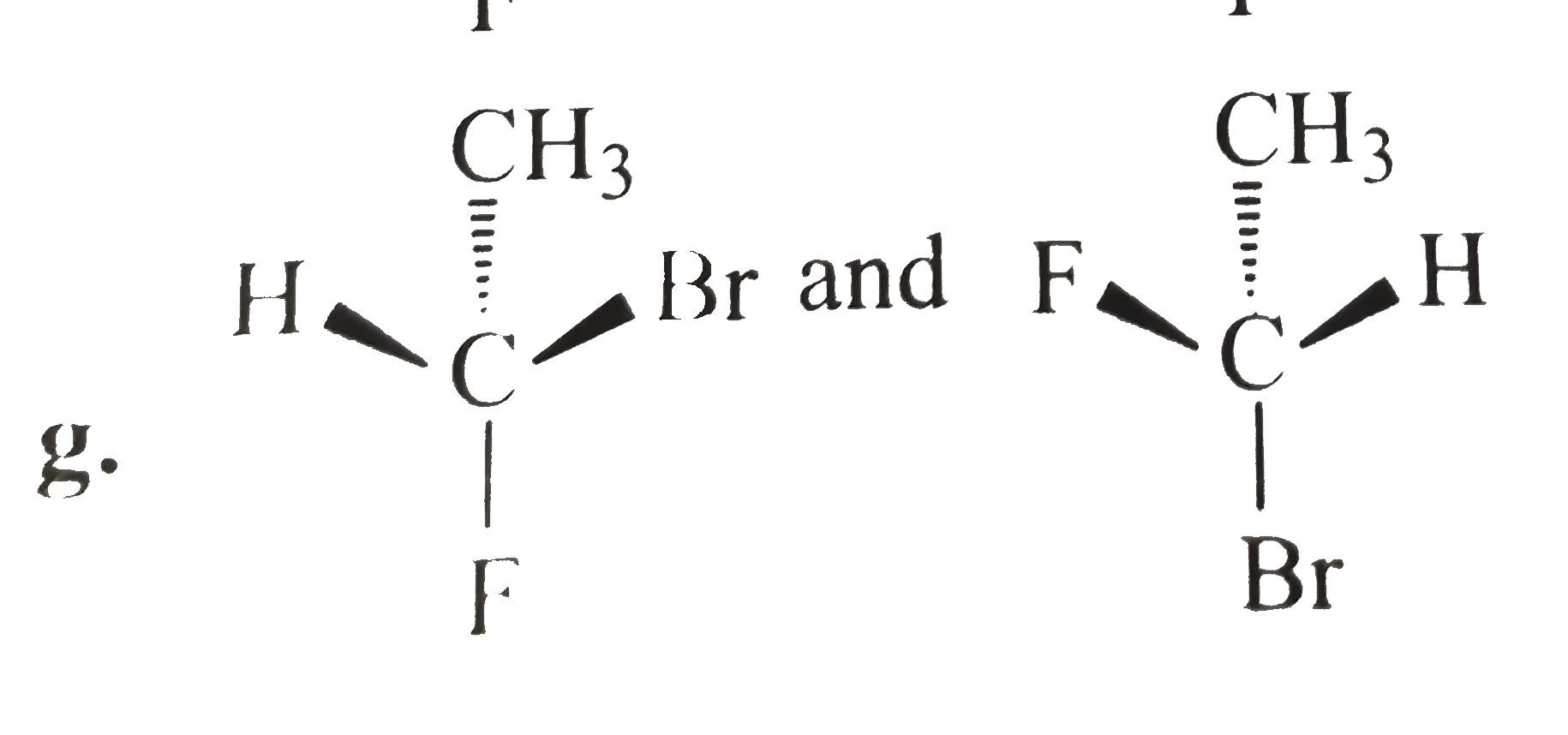
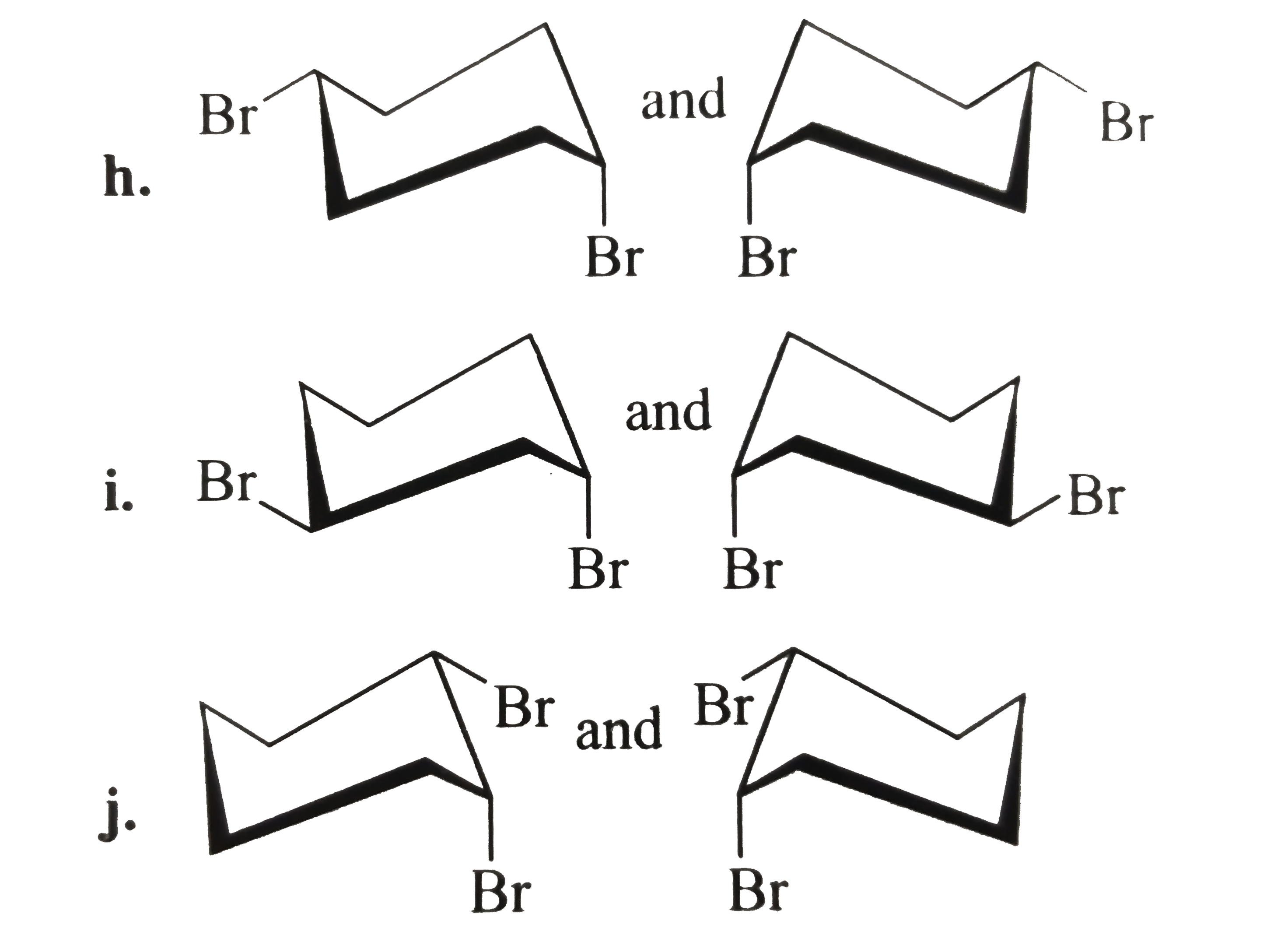
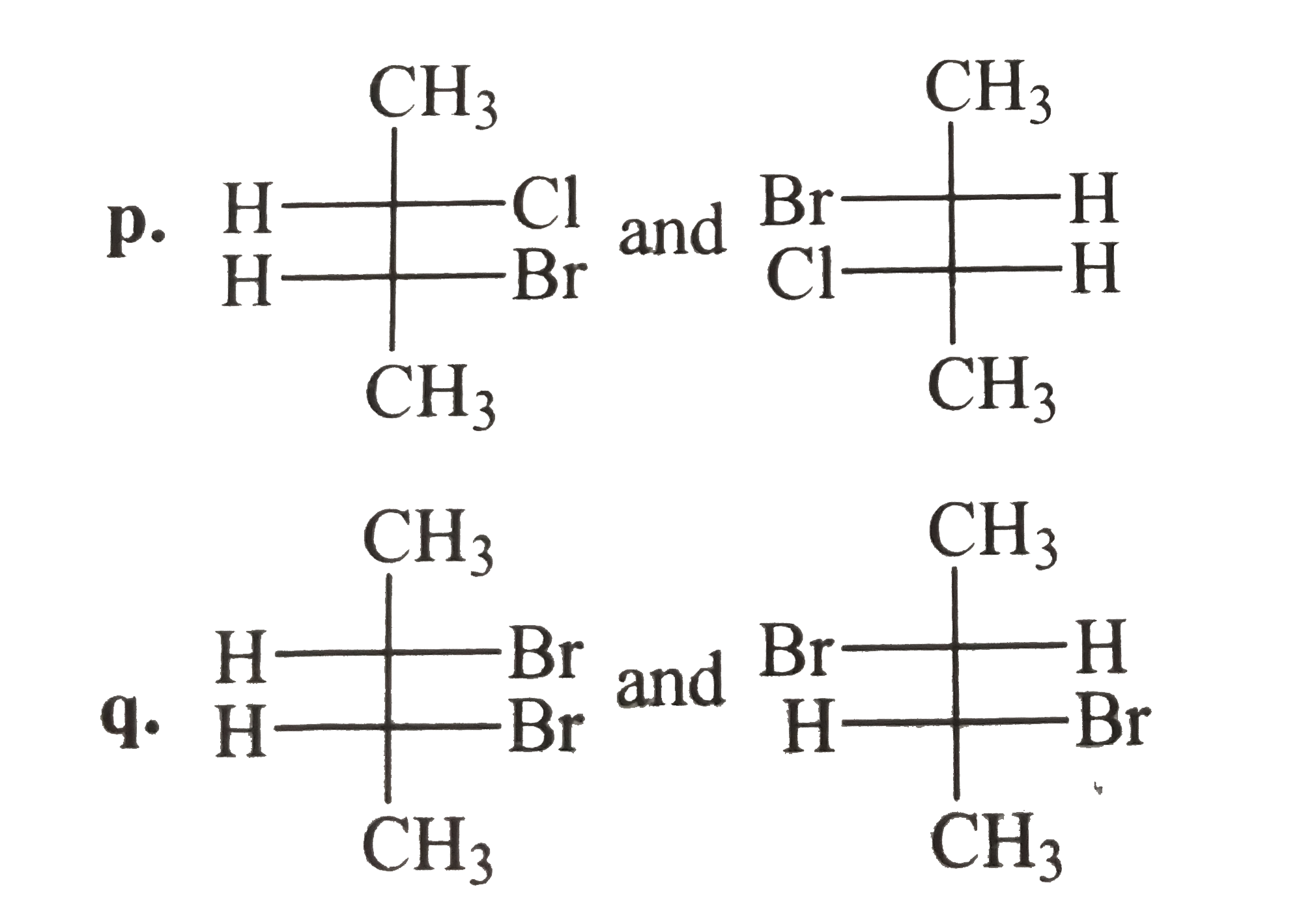
- Tell whether the two structrues in each pair represent enantiomers or ...
Text Solution
|
- A sample of optically active alochol has a specific ratation, [alpha](...
Text Solution
|
- The following are the formule for three compounds 2,3- dichlorobutane ...
Text Solution
|
- Write the three-dimensional formulas for all of the stereoisomers of e...
Text Solution
|
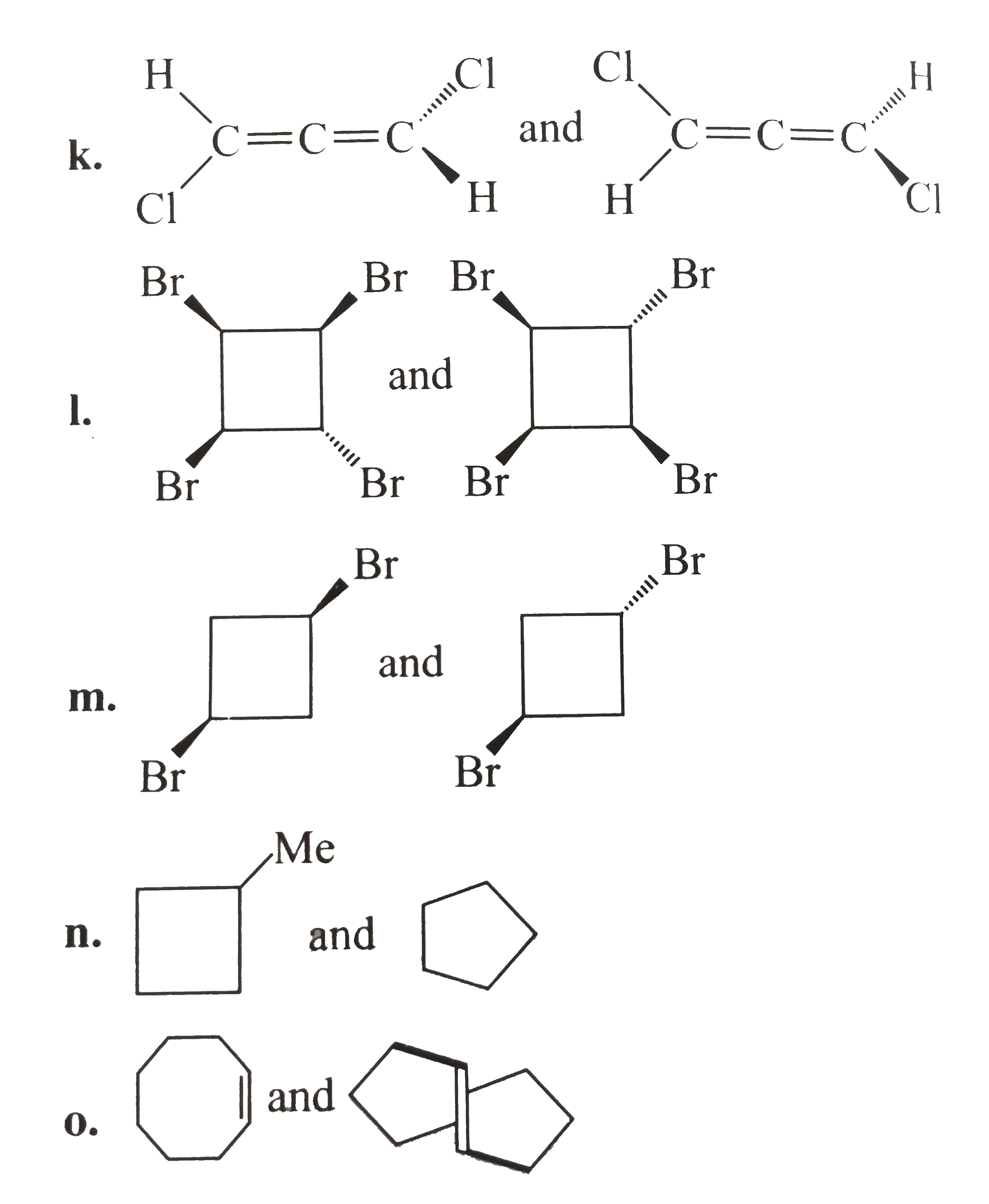
- Consider the following pairs of structrue. Identify the relationship b...
Text Solution
|
- Discuss the anticipated stereochemistry of each of the following compo...
Text Solution
|
- Write formulae for all the isomers of each of the following. Designate...
Text Solution
|
- Which of the following are chiral and capable of existing as enantiome...
Text Solution
|
- a. Write the structrue of 2,2- dibromobicyclo [2.2.1] heptane. b. How...
Text Solution
|