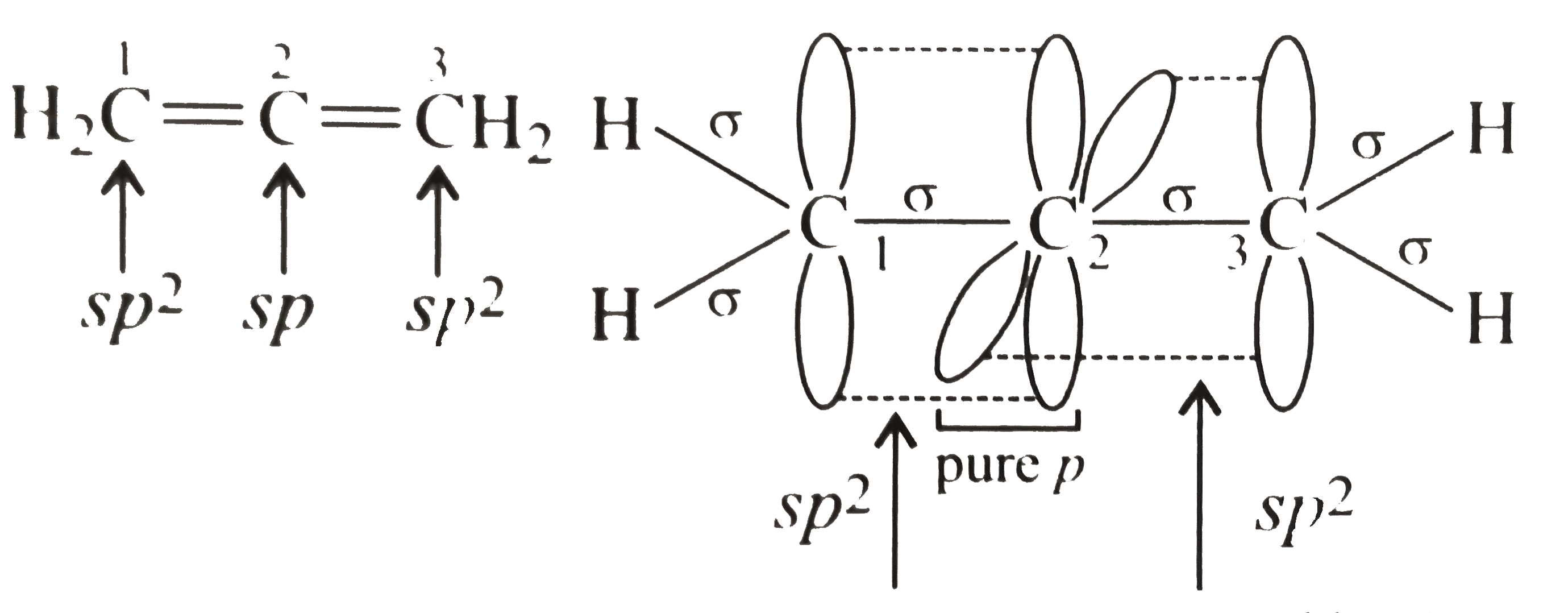Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-ISOMERISM-Analytical and Descriptive Type (Exercise)
- Write structural formula for all the isomeric alcohols having the mole...
Text Solution
|
- Write the structural of all the possible isomers of dichloroethene. Wh...
Text Solution
|
- Write tautomeric form of phenol
Text Solution
|
- Discuss the hybridisation of carbon atoms in allene (C(3)H(4)) and sho...
Text Solution
|
- Identify the pairs of enantiomers and diastereomers from the following...
Text Solution
|
- Draw Newman projection of the less stable staggered from of butane. ...
Text Solution
|