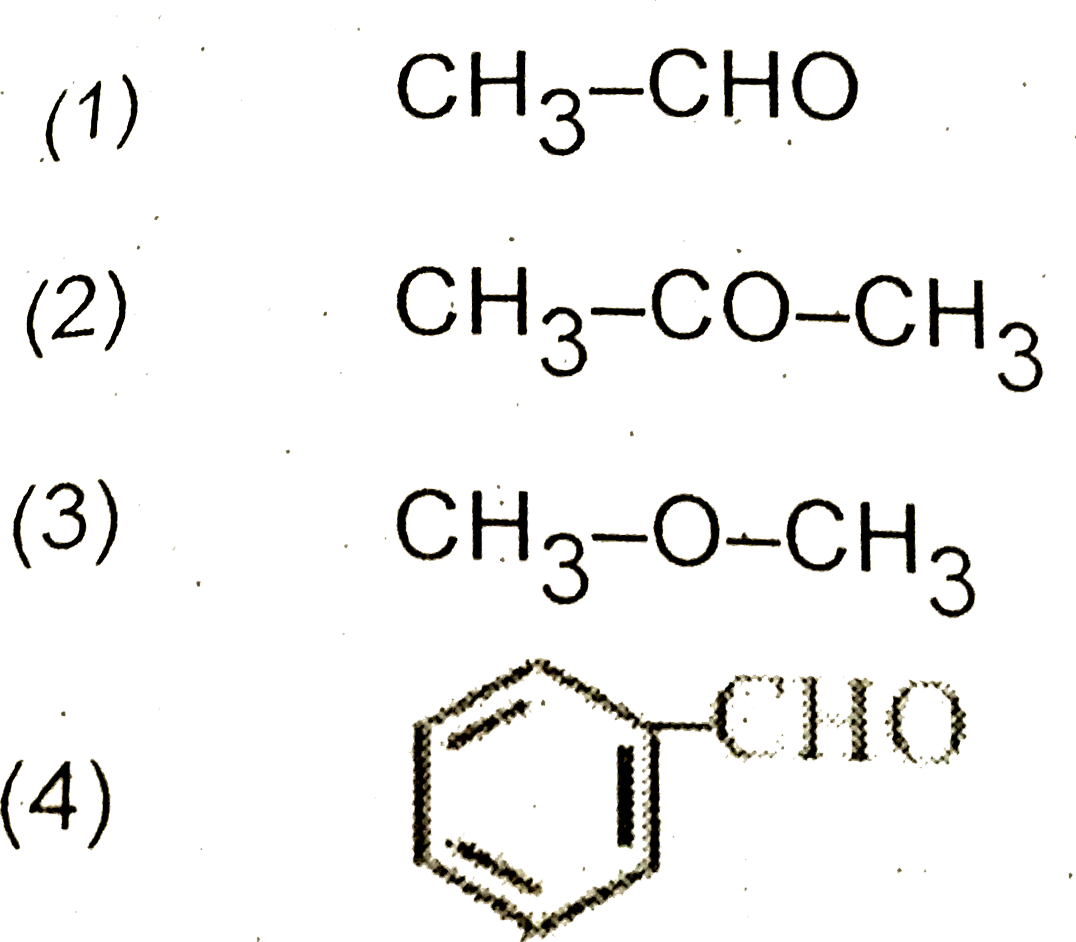
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the following will not give D.N.P test ?
Text Solution
|
- Which of the following will not give the iodoform test?
Text Solution
|
- Which of the following will give carbylamine test?
Text Solution
|
- Which of the following gives iodoform test ?
Text Solution
|
- Which of the following gives Fehling's test ?
Text Solution
|
- Given reason for the following : (i) Glucose does not give 2, 4 D.N.P....
Text Solution
|
- which of the following gives iodoform test ?
Text Solution
|
- Which of the following give isocyanide test?
Text Solution
|
- Which of the following will not give D.N.P test ?
Text Solution
|