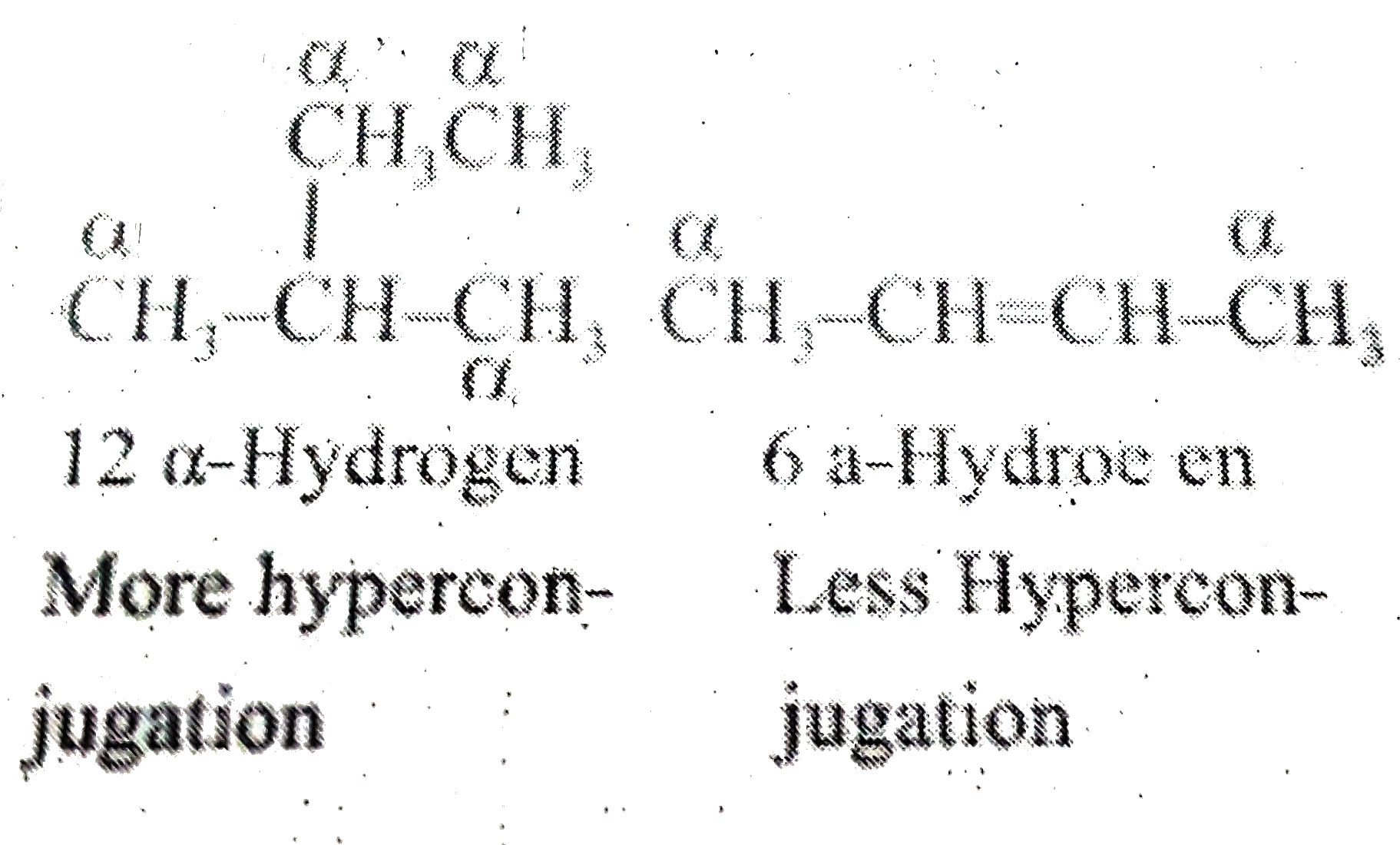A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The effect that makes 2, 3–dimethyl-2-butene more stable than 2-butene...
Text Solution
|
- The decreasing order of reactivity of the following alkenes is: i. 2...
Text Solution
|
- Assertion : 2- Bromobutane on reaction with sodium ethoxide in ethanol...
Text Solution
|
- Assertion(A):2-Bromobutane on reaction with sodium ethoxide. In ethano...
Text Solution
|
- (A) 2- Bromobutane on reaction with sodium ethoxide in ethanol gives 1...
Text Solution
|
- The effect that makes 2,3-dimethyl-2-butene more stable than 2-butene ...
Text Solution
|
- Assertion : 2-Bromobutane on reaction with sodium ethoxide in ethanol ...
Text Solution
|
- The effect that makes 2, 3-dimethyl-2-butene more stable than 2-butene...
Text Solution
|
- Assertion:2-Bromobutane on reaction with sodium ethoxide in ethanol gi...
Text Solution
|