Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
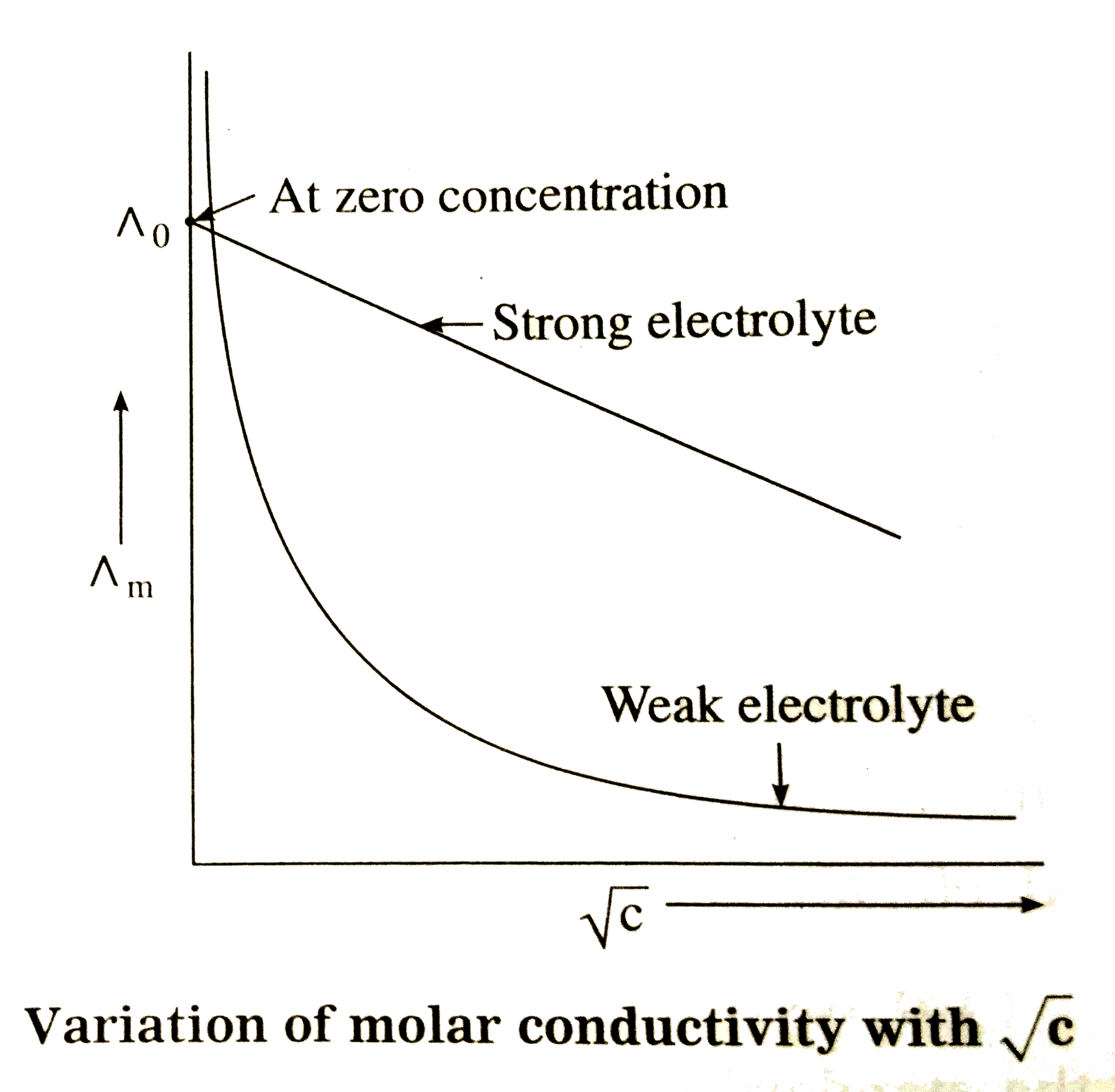
- Explain the variation of molar conductivity with concentration for str...
Text Solution
|
- For a dilute solution of a strong electrolyte, the variation of molar ...
Text Solution
|
- Define molar conductivity of a solution and explain how molar conducti...
Text Solution
|
- Explain with a graph the variation of molar conductivity of a strong e...
Text Solution
|
- Explain the variation of molar conductivity with concentration for str...
Text Solution
|
- How is molar conductivity related to concentration of the electrolyte ...
Text Solution
|
- Explain with a graph the variation of molar conductivity of a strong e...
Text Solution
|
- How does molar conductivity vary with concentration for weak and stron...
Text Solution
|
- How does the variation in molar conductivity of an electrolyte with co...
Text Solution
|