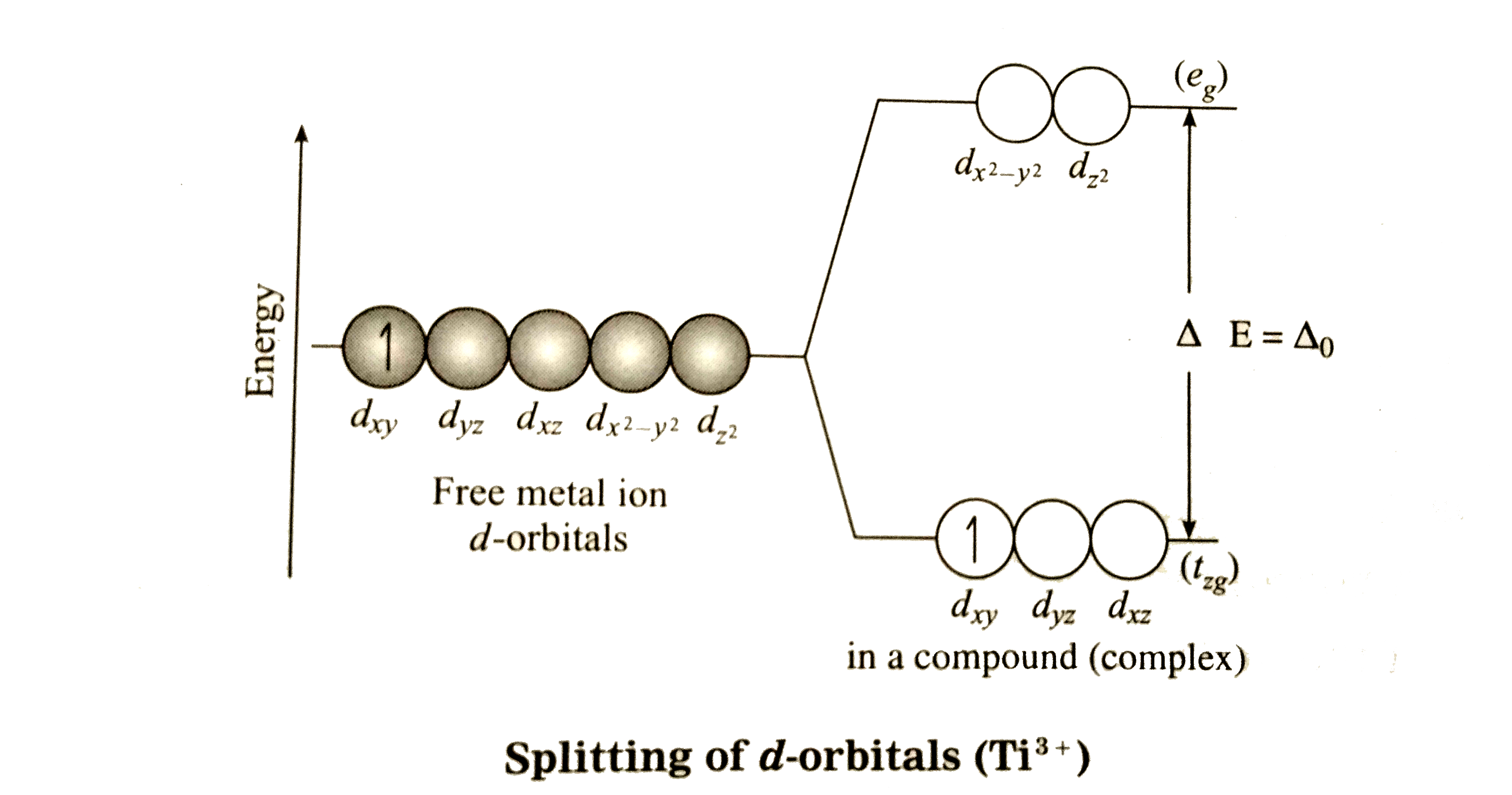
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- How does a transition metal ion acquire a colour?
Text Solution
|
- The colour of the transition metal ions is due to
Text Solution
|
- The colour of transition metal ion is attributed to:
Text Solution
|
- The colour of the transition metal ions is//are due to:
Text Solution
|
- The colour of the transition metal ions is due to
Text Solution
|
- The colour of the transition metal ions is due to
Text Solution
|
- d-d संक्रमण क्या है तथा यह संक्रमण धातु आयनों को किस प्रकार रंग प्रदान...
Text Solution
|
- How does a transition metal ion acquire a colour?
Text Solution
|
- The colour of transition metal ions is due to
Text Solution
|