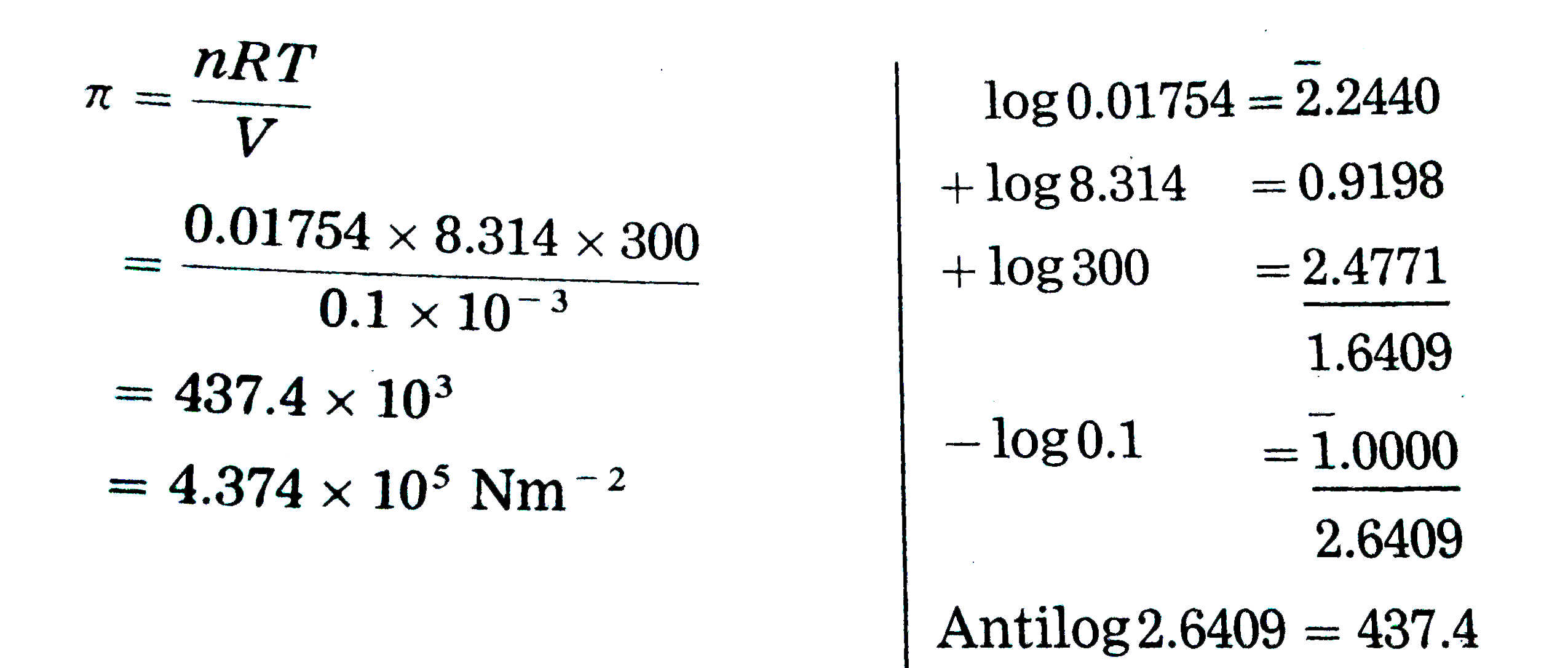Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Calculate the osmotic pressure of 6% sucrose (C(12) H(22) O(11))...
Text Solution
|
- Calculate the osmotic pressure of 5% solution of cane sugar (sucrose) ...
Text Solution
|
- Given : 1 L, 2M solution of C(12)H(22)O(11) under go first order react...
Text Solution
|
- Calculate the osmotic pressure of the solution prepared in the above q...
Text Solution
|
- The heat of combustion of sucrose C(12) H(22) O(11)(s) at constant vol...
Text Solution
|
- The oxidation number of carbon in C(12) H(22) O(11) is
Text Solution
|
- The osmotic pressure of 0.2 molar solution of urea at 300 K(R = 0.082)...
Text Solution
|
- Calculate the osmotic pressure of 0.2 M glucose solution at 300 ...
Text Solution
|
- Calculate the osmotic pressure of 6% sucrose (C(12) H(22) O(11))...
Text Solution
|