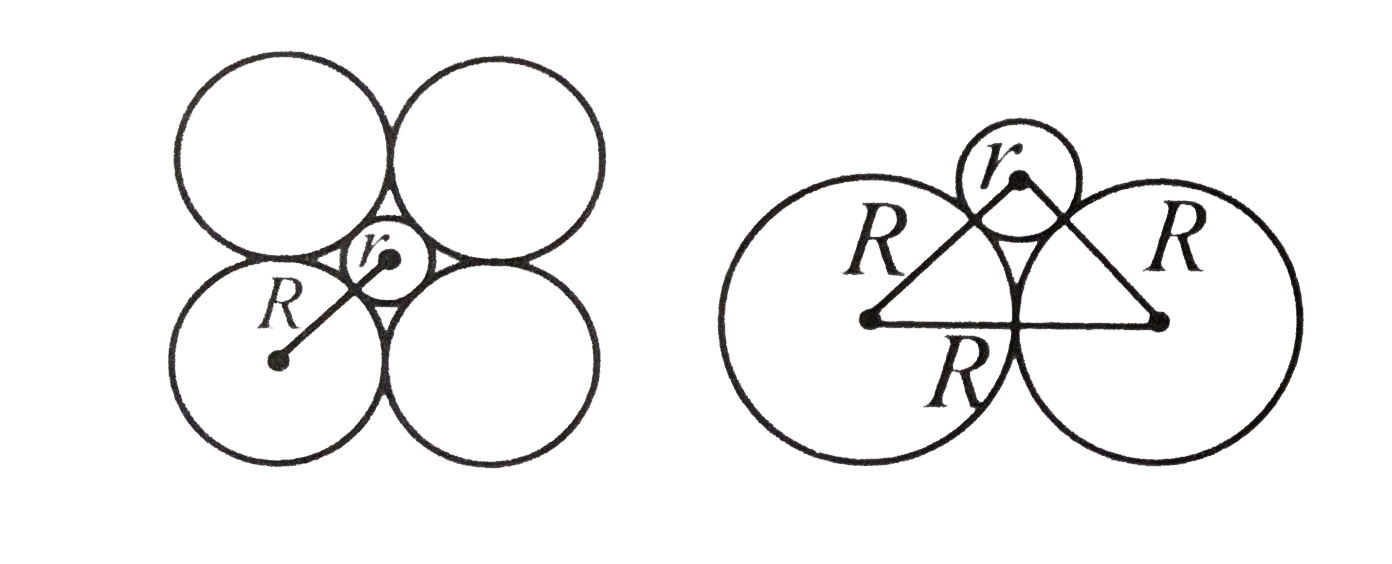A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THE SOLID STATE
NCERT FINGERTIPS|Exercise Packing Efficiency|13 VideosTHE SOLID STATE
NCERT FINGERTIPS|Exercise Calculations Involving Unit Cell Dimensions|9 VideosTHE SOLID STATE
NCERT FINGERTIPS|Exercise Crystalline And Unit Cells|6 VideosTHE P-BLOCK ELEMENTS
NCERT FINGERTIPS|Exercise Assertion And Reason|15 Videos
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS-THE SOLID STATE -Number Of Atoms In Unit Cell And Close Packed Structures
- Which of the following statements is not about the voids ?
Text Solution
|
- If 'Z' is the number of atoms in the unit cell that represents the cl...
Text Solution
|
- If the radius of the octaheral void is r and the radius of the atoms i...
Text Solution
|
- A metallic crystal cystallizes into a lattice containing a sequence of...
Text Solution
|
- In ccp arrangement the pattern of successive layers can be designated ...
Text Solution
|
- which of the following statemets is not true about the hexagona...
Text Solution
|
- The cordination number of a metal crystallising in a hexagonal close-p...
Text Solution
|
- Match the column I with Column II and mark the appropriate choice .
Text Solution
|
- IF the radius ratio of cation to anion is in the range of 0.2...
Text Solution
|
- A crystal lattice with alternate +ve "and "-veions has radius ratio o...
Text Solution
|
- A solid AB has the NaCL structure, If radius of cation A^(+) is 120 pm...
Text Solution
|
- A crystal is formed by two elements X and Y in cubic structure. X atom...
Text Solution
|
- If there elements X, Y & Z crystallize in cubic solid lattice with X- ...
Text Solution
|
- A compound formed by two elements M and N. Element N forms ccp and ato...
Text Solution
|
- A cubic solid is made up iof two elements X and Y . Atoms Y are presen...
Text Solution
|
- How many chloride ions are there around sodium ion in sodium chloride ...
Text Solution
|
- In NaCl structure , all the :
Text Solution
|
- 8:8 coordination of CsCl is found to change into 6:6 coordination :
Text Solution
|
- In CsCI lattice the coordination number of Cs ion is
Text Solution
|
- Which is /are correct statement about zinc blende structure ?
Text Solution
|