A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SOLUTIONS
NCERT FINGERTIPS|Exercise Vapour Pressure Of Liquid Solutions|5 VideosSOLUTIONS
NCERT FINGERTIPS|Exercise MCQs|2 VideosSOLUTIONS
NCERT FINGERTIPS|Exercise Expressing Concentration Of Solutions|16 VideosPRACTICE PAPER -3
NCERT FINGERTIPS|Exercise Practice Paper 3|50 VideosSURFACE CHEMISTRY
NCERT FINGERTIPS|Exercise Assertion And Reason|15 Videos
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS-SOLUTIONS -Solubility
- Solubility of a substance is its maximum amount that can be dissolved ...
Text Solution
|
- During dissolution when solute is added to the solvent, some solute pa...
Text Solution
|
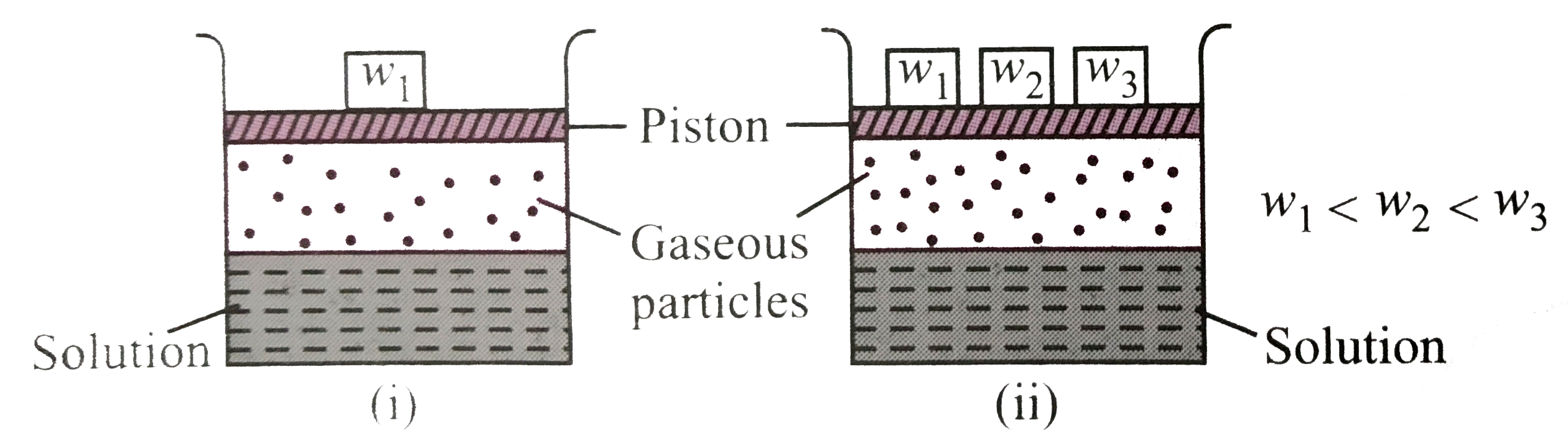
- Consider the two figures given below : Which of the following sta...
Text Solution
|
- The law which indicates the relationship between solubility of a gas i...
Text Solution
|
- According to Henry's law the partial pressure of the gas in vapour pha...
Text Solution
|
- The value of Henry's law constant for some gases at 293 K is given bel...
Text Solution
|
- H(2)S, a toxic gas with rotten egg like smell, is used for the qualita...
Text Solution
|
- Henry's law constant for the molality of methane in benzene at 298K is...
Text Solution
|
- When a gas is bubbled through water at 298 K, a very dilute solution o...
Text Solution
|
- Henry's law constant of oxygen is 1.4 xx 10^(-3) mol "lit"^(-1) "atm"^...
Text Solution
|
- At high altitudes the partial pressure of oxygen is less than that at ...
Text Solution
|