A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SOLUTIONS
NCERT FINGERTIPS|Exercise Abnormal Molar Masses|16 VideosSOLUTIONS
NCERT FINGERTIPS|Exercise Higher Order Thinking Skills|10 VideosSOLUTIONS
NCERT FINGERTIPS|Exercise Ideal And Non-Ideal Solutions|13 VideosPRACTICE PAPER -3
NCERT FINGERTIPS|Exercise Practice Paper 3|50 VideosSURFACE CHEMISTRY
NCERT FINGERTIPS|Exercise Assertion And Reason|15 Videos
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS-SOLUTIONS -Colligative Properties And Determination Of Molar Mass
- Vapour pressure of a pure liquid X is 2 atm at 300 K. It is lowered to...
Text Solution
|
- An aqueous solution of 2 per cent (wt.//wt) non-volatile solute exerts...
Text Solution
|
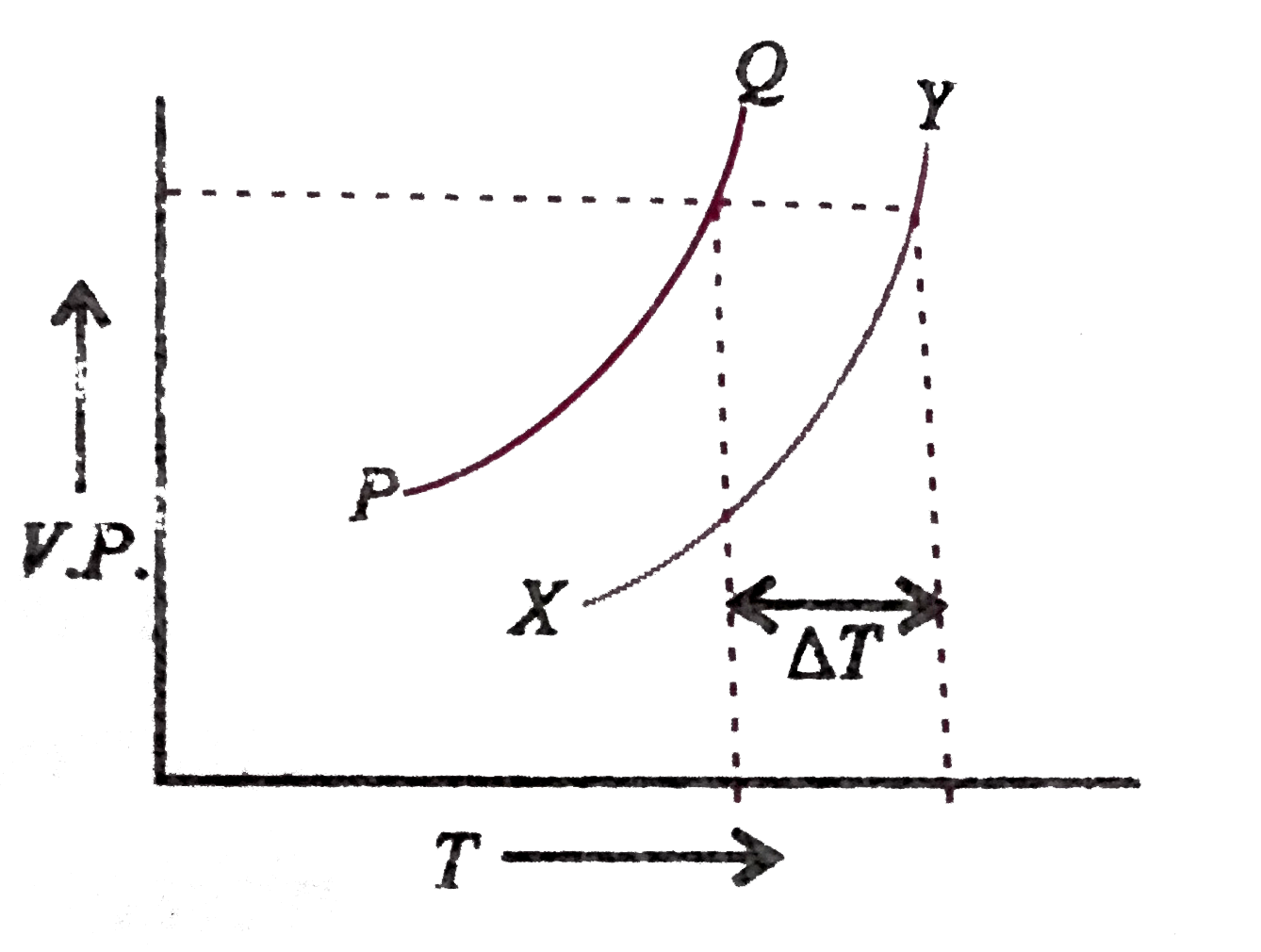
- In the graph plotted between vapour pressure (V.P.) and temperature (T...
Text Solution
|
- A solution containing 12.5 g of non-electrolyte substance in 185 g of ...
Text Solution
|
- If 1 g of solute (molar mass = 50 g mol^(-1)) is dissolved in 50 g of ...
Text Solution
|
- 2 g of sugar is added to one litre of water to give sugar solution. Wh...
Text Solution
|
- How does sprinking of salt help in clearing the snow covered roads in ...
Text Solution
|
- Equimolar solutions in the same solvent have-
Text Solution
|
- A 5% solution (w/W) of cane sugar (molar mass = 342 g mol^(-1) ) has f...
Text Solution
|
- What weight of glycerol should be added to 600 g of water in order to ...
Text Solution
|
- If semipermeable membrane is placed between the solvent and solution a...
Text Solution
|
- Study the following figure showing osmosis and mark the correct statem...
Text Solution
|
- Relative lowering of vapour pressure , osmotic pressure of a solution ...
Text Solution
|
- The osmotic pressure of a solution can be increased by
Text Solution
|
- People taking lot of salt experience puffiness or swelling of the body...
Text Solution
|
- The preservation of meat by salting and of fruits by adding sugar prot...
Text Solution
|
- Sea water is desalinated to get fresh water by which of the following ...
Text Solution
|
- A 5% solution of cane sugar (molecular weight=342) is isotonic with 1%...
Text Solution
|
- Which of the following statements is correct about diffusion and osmos...
Text Solution
|
- 10% solution of urea is isotonic with 6% solution of a non-volatile so...
Text Solution
|