A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL KINETICS
NCERT FINGERTIPS|Exercise MCQs|3 VideosCHEMICAL KINETICS
NCERT FINGERTIPS|Exercise Integrated Rate Equation|25 VideosCHEMICAL KINETICS
NCERT FINGERTIPS|Exercise Assertion And Reason|15 VideosBIOMOLECULES
NCERT FINGERTIPS|Exercise Assertion And Reason|15 VideosCHEMISTRY IN EVERYDAY LIFE
NCERT FINGERTIPS|Exercise Assertion And Reason|15 Videos
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS-CHEMICAL KINETICS-Factors Influencing Rate Of Reaction
- Which of the following is an example of a fractional order reaction?
Text Solution
|
- What will be the rate equation for the reaction 2X + Y to Z, if the or...
Text Solution
|
- For a reaction, I^(-) + OCl^(-) to IO^(-) + Cl^(-) in an aqueous mediu...
Text Solution
|
- For a reaction, 2NO + 2H(2) to N(2) + 2H(2) O, the possible mechanism ...
Text Solution
|
- Rate constant of two reactions are given below. Identifying their orde...
Text Solution
|
- Find the values of A,B and C in the following table for the reaction X...
Text Solution
|
- For a chemical reaction A rarr B, the rate of reaction increases by a ...
Text Solution
|
- For a reaction AtoB, the rate of reaction becomes twenty seven times w...
Text Solution
|
- For the reaction A + B to products, what will be the order of reaction...
Text Solution
|
- The unit of rate constant for the reaction, 2H(2) + 2NO to 2H(2)O + ...
Text Solution
|
- Match the rate law given in column I with the dimensions of rate const...
Text Solution
|
- The decomposition of dimethyl ether is a fractional order reaction. Th...
Text Solution
|
- The unit of rate of reaction and rate of rate constant are same for a ...
Text Solution
|
- The rate of the reaction: CH(3)COOC(2)H(5) + NaOH to Ch(3)COONa + C(...
Text Solution
|
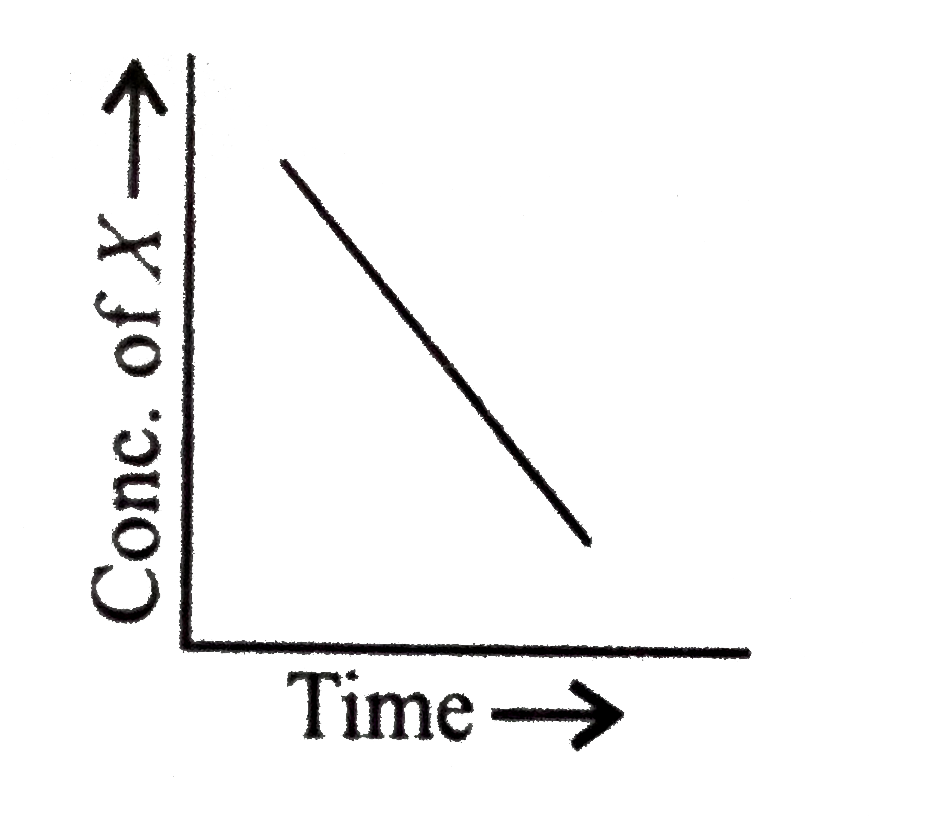
- For a general reaction X to Y, the plot of conc. Of X vs Time is giv...
Text Solution
|
- Fill in the blanks by choosing the correct option. Order of the reacti...
Text Solution
|
- The number of molecules of the reactants taking part in a single step ...
Text Solution
|
- In any unimolecular reaction
Text Solution
|
- The overall rate of a reaction is governed by :
Text Solution
|
- For a reaction X + Y to Z, rate prop [X]. What is (i) molecularity and...
Text Solution
|