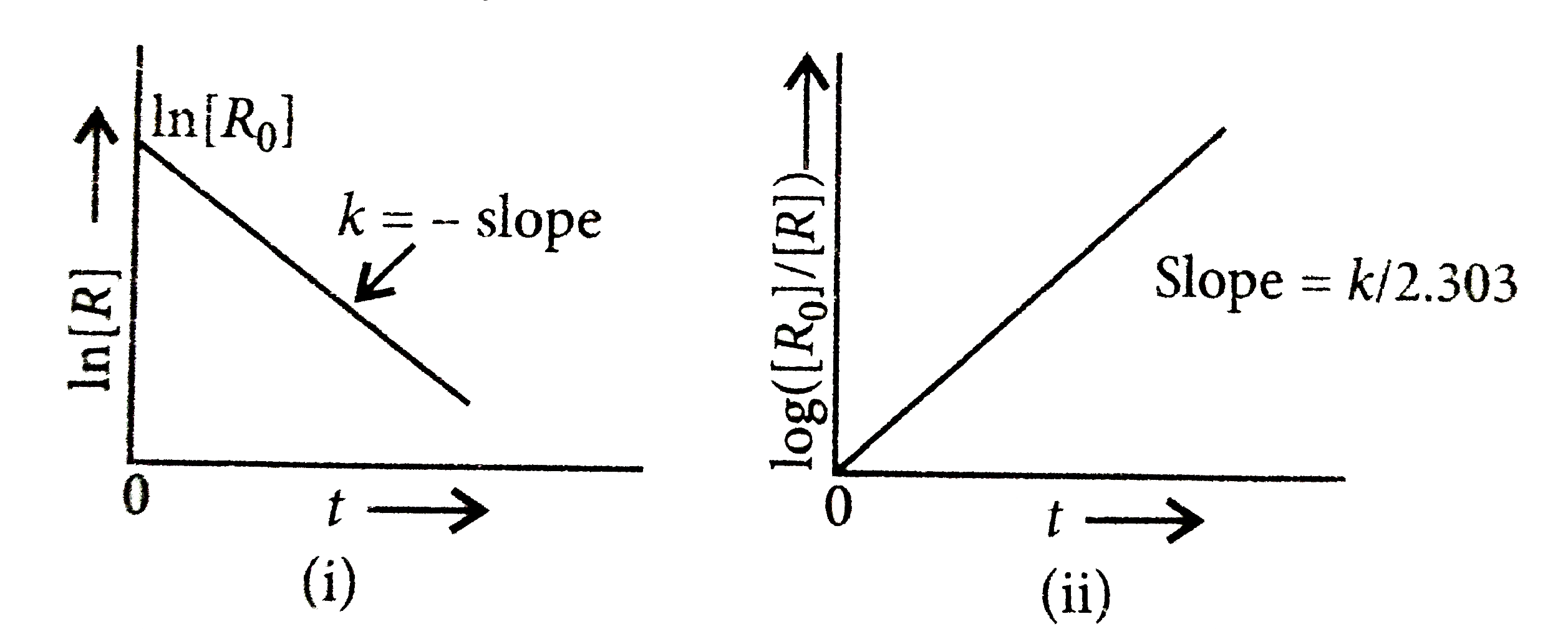
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL KINETICS
NCERT FINGERTIPS|Exercise Temperature Dependendence Of The Rate Of A Reaction|17 VideosCHEMICAL KINETICS
NCERT FINGERTIPS|Exercise Collision Theory Of Chemical Reactions|6 VideosCHEMICAL KINETICS
NCERT FINGERTIPS|Exercise MCQs|3 VideosBIOMOLECULES
NCERT FINGERTIPS|Exercise Assertion And Reason|15 VideosCHEMISTRY IN EVERYDAY LIFE
NCERT FINGERTIPS|Exercise Assertion And Reason|15 Videos
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS-CHEMICAL KINETICS-Integrated Rate Equation
- Derive an expression to calculate time required for completion of zero...
Text Solution
|
- A plot of log (a-x) against time 't' is a straight line. This indicate...
Text Solution
|
- Observe the given graphs carefully. Which of the given orders are...
Text Solution
|
- Radioactive distintegration is an example of
Text Solution
|
- A first order reaction has a rate constant of 5 xx 10^(-3) s^(-1). How...
Text Solution
|
- In a first order reaction, the concentration of reactant decrease from...
Text Solution
|
- A first order reaction is 20% complete in 10 minutes. What is the spec...
Text Solution
|
- The decomposition of a substance follows first order kinetics. If its ...
Text Solution
|
- A first order reaction takes 40 min for 30% decomposition. Calculate t...
Text Solution
|
- The following data were obtained during the first order thermal decomp...
Text Solution
|
- A first order reaction has a rate constant 1.15xx10^(-3)s^(-1). How lo...
Text Solution
|
- The decomposition of dinitrogen pentoxide (N(2)O(5)) follows first ord...
Text Solution
|
- Half life of a first order reaction in 10 min. What % of reaction will...
Text Solution
|
- The half life for radioactive decay of .^(14)C is 5730 years. An archa...
Text Solution
|
- What will be the half-life of the first order reaction for which the v...
Text Solution
|
- The rate constant of first order reaction is 10^(-2)"min"^(-1). The ha...
Text Solution
|
- In a first order reaction, The concentration of reactant is reduced to...
Text Solution
|
- Calculate the half life of the reaction ArarrB, when the initial conce...
Text Solution
|
- In pseudo-unimolecular reactions :
Text Solution
|
- The value of rate of a pseudo first order reaction depends upon
Text Solution
|