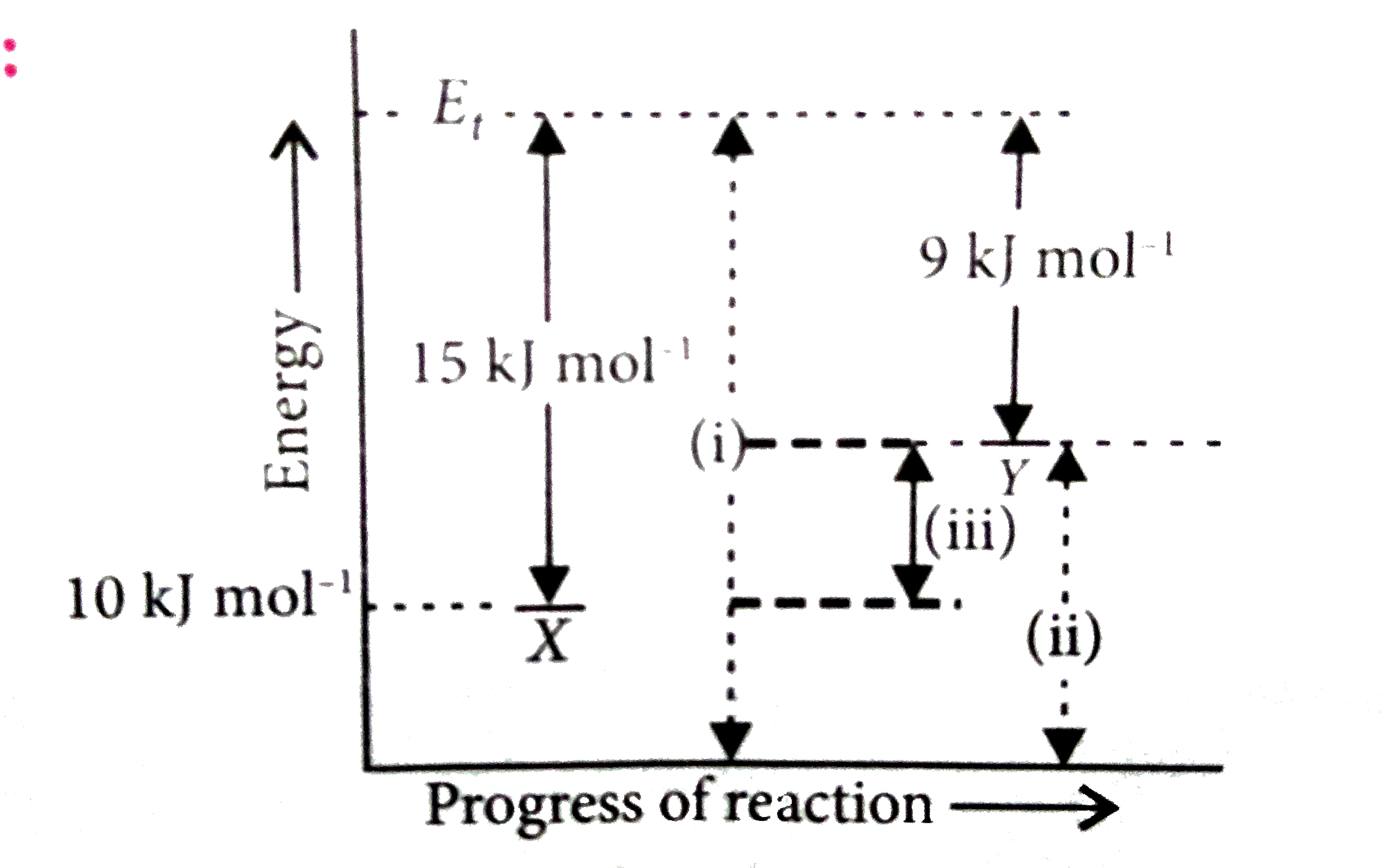
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL KINETICS
NCERT FINGERTIPS|Exercise NCERT Exemplar|20 VideosCHEMICAL KINETICS
NCERT FINGERTIPS|Exercise Assertion And Reason|15 VideosCHEMICAL KINETICS
NCERT FINGERTIPS|Exercise Collision Theory Of Chemical Reactions|6 VideosBIOMOLECULES
NCERT FINGERTIPS|Exercise Assertion And Reason|15 VideosCHEMISTRY IN EVERYDAY LIFE
NCERT FINGERTIPS|Exercise Assertion And Reason|15 Videos
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS-CHEMICAL KINETICS-Higher Order Thinking Skills
- The reaction 2NO + Br(2) to 2NOBr, obeys the following mechanism: NO...
Text Solution
|
- A hypothetical reaction A(2) + B(2) rarr 2AB follows the mechanism as ...
Text Solution
|
- In acidic medium the rate of reaction between [BrO(3)^(-)] and [Br^(-)...
Text Solution
|
- The rate of certain hypothetical reaction A+B+C rarr Products, is gi...
Text Solution
|
- For the reaction, H(2(g)) + Br(2(g)) to 2HBr((g)), the reaction rate =...
Text Solution
|
- For a first order reaction, the ratio of the time taken for 7//8^(th) ...
Text Solution
|
- A first order reaction is 50% completed in 30 min at 27^(@)C and in 10...
Text Solution
|
- An exothermic chemical reaction proceeds by two stages reactants over...
Text Solution
|
- In a hypothetical reaction X to Y, the activation energy for the forwa...
Text Solution
|