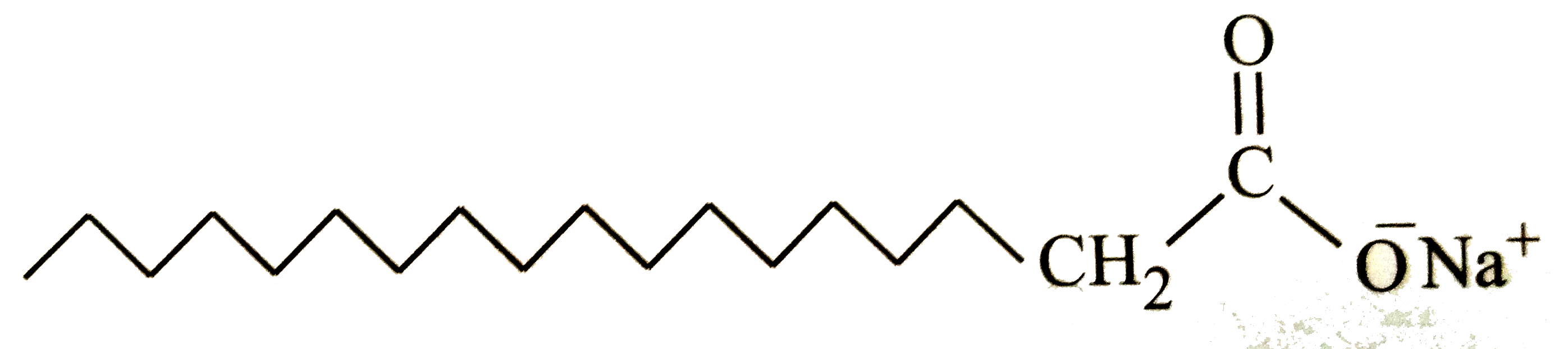
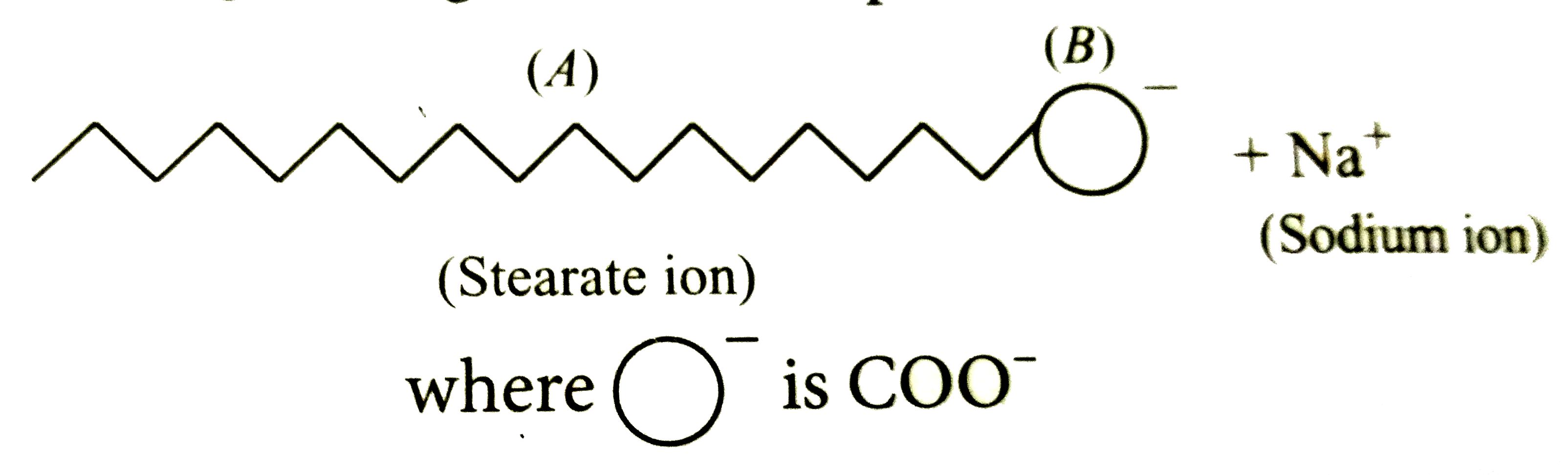A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SURFACE CHEMISTRY
NCERT FINGERTIPS|Exercise MCQS|2 VideosSURFACE CHEMISTRY
NCERT FINGERTIPS|Exercise Emulsions|7 VideosSURFACE CHEMISTRY
NCERT FINGERTIPS|Exercise Catalysis|14 VideosSOLUTIONS
NCERT FINGERTIPS|Exercise Assertion And Reason|15 VideosTHE D- AND F- BLOCK ELEMENTS
NCERT FINGERTIPS|Exercise Assertion And Reason|15 Videos
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS-SURFACE CHEMISTRY-Colloids And Classification Of Colloids
- CMC (Critical Micelle Concentration) is
Text Solution
|
- At the critical micelle concentration, the surfactant molecules :
Text Solution
|
- In the given figure label the parts
Text Solution
|
- Soap mixed with water below critical micelle concentration behaves as
Text Solution
|
- White of an egg whipped with water acts as
Text Solution
|
- Colloidal solutions of metals like gold can be prepared when their sal...
Text Solution
|
- When a small quantity of FeCl(3) solution is added to the fresh precip...
Text Solution
|
- Which of the processes is being shown in the figure ?
Text Solution
|
- Which of the following is not a method of removing impurities from a c...
Text Solution
|
- Tyndall effect is observed only when (i) the diameter of the dispers...
Text Solution
|
- When a colloidal solution is viewed from the direction at right angles...
Text Solution
|
- Tyndall effect is not observed in
Text Solution
|
- Which of the following systems will show Tyndall effect ?
Text Solution
|
- Why is ferric hydroxide colloid positively charged when prepared by ad...
Text Solution
|
- When an excess of a very dilute aqueous solution of KI is addd to a ve...
Text Solution
|
- The ratio of the number of moles of AgNO(3), Pb(NO(3))(2) and Fe(NO(3)...
Text Solution
|
- The combination of two layers of opposite charges aroung the colloidal...
Text Solution
|
- Which of the following is not an explanation for the origin of charge ...
Text Solution
|
- Movement of dispersion medium under the influence of electric field is...
Text Solution
|
- Why is alum added to water containing suspended impurities ?
Text Solution
|