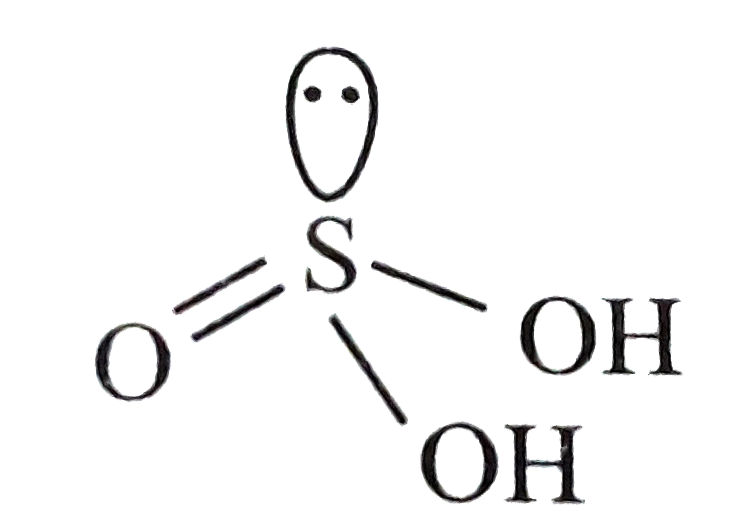
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THE P-BLOCK ELEMENTS
NCERT FINGERTIPS|Exercise Sulphuric Acids|4 VideosTHE P-BLOCK ELEMENTS
NCERT FINGERTIPS|Exercise Group 17 Elements - The Halogen Family|10 VideosTHE P-BLOCK ELEMENTS
NCERT FINGERTIPS|Exercise Sulphur Dioxide|4 VideosTHE D- AND F- BLOCK ELEMENTS
NCERT FINGERTIPS|Exercise Assertion And Reason|15 VideosTHE SOLID STATE
NCERT FINGERTIPS|Exercise Assertion And Reason|15 Videos
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS-THE P-BLOCK ELEMENTS -Oxoacids Of Sulphur
- Which of the following statements is not correct?
Text Solution
|
- The oxyacid of sulphur that contains a lone pair of electrons in sulph...
Text Solution
|
- In which of the following sulphur is present in +5 oxidation state?
Text Solution
|
- The oxidation states of sulphur in the anions SO(3)^(2-), S(2)O(4)^(2-...
Text Solution
|
- The hybridisation state of the central atom and shape of the molecules...
Text Solution
|
- Match the column I with column II and mark the appropriate choice.
Text Solution
|
- Match the column I with column II and mark the appropriate choice.
Text Solution
|