A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THE P-BLOCK ELEMENTS
NCERT FINGERTIPS|Exercise Higher Order Thinking Skills|7 VideosTHE P-BLOCK ELEMENTS
NCERT FINGERTIPS|Exercise NCERT Exemplar|27 VideosTHE P-BLOCK ELEMENTS
NCERT FINGERTIPS|Exercise Interhalogen Compounds|2 VideosTHE D- AND F- BLOCK ELEMENTS
NCERT FINGERTIPS|Exercise Assertion And Reason|15 VideosTHE SOLID STATE
NCERT FINGERTIPS|Exercise Assertion And Reason|15 Videos
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS-THE P-BLOCK ELEMENTS -Group 18 Elements - The Noble Gases
- Xenon has closed shell configuration but is known to give compounds wi...
Text Solution
|
- Which compound is prepared by the following reaction ? underset(("2:...
Text Solution
|
- Which of the following statements is not correct about XeF(2)?
Text Solution
|
- In the clathrates of xenon with water the nature of bonding in Xe and ...
Text Solution
|
- Complete the following reactions by filling the appropriate choice. ...
Text Solution
|
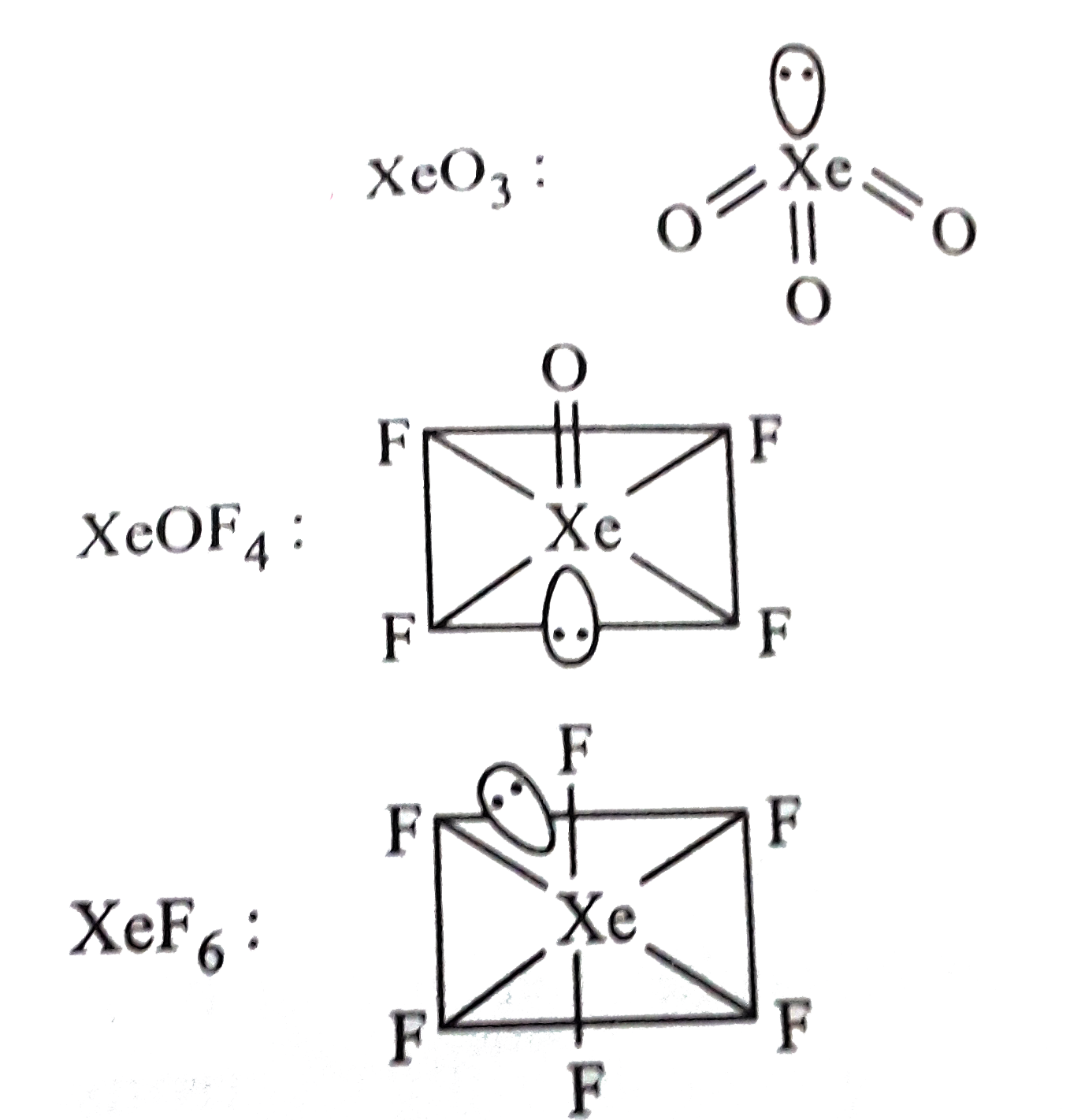
- Among the following molecules (i) XeO(3) (ii) XeOF(4) (iii) XeF(6) ...
Text Solution
|
- Match the column I with column II and mark the appropriate choice.
Text Solution
|
- Match the list of noble gas compounds in column I with their shapes in...
Text Solution
|
- In XeF(2),XeF(4)" and "XeF(6), the number of the lone pairs of Xe resp...
Text Solution
|
- Oganesson has been synthetically produced by collision of
Text Solution
|
- Which of the following is not correct about xenon hexafluoride?
Text Solution
|
- Fill in the blanks by choosing the appropriate option. The noble gases...
Text Solution
|
- Which of the following is not a use of noble gases?
Text Solution
|
- Which of the following statements is not correct?
Text Solution
|