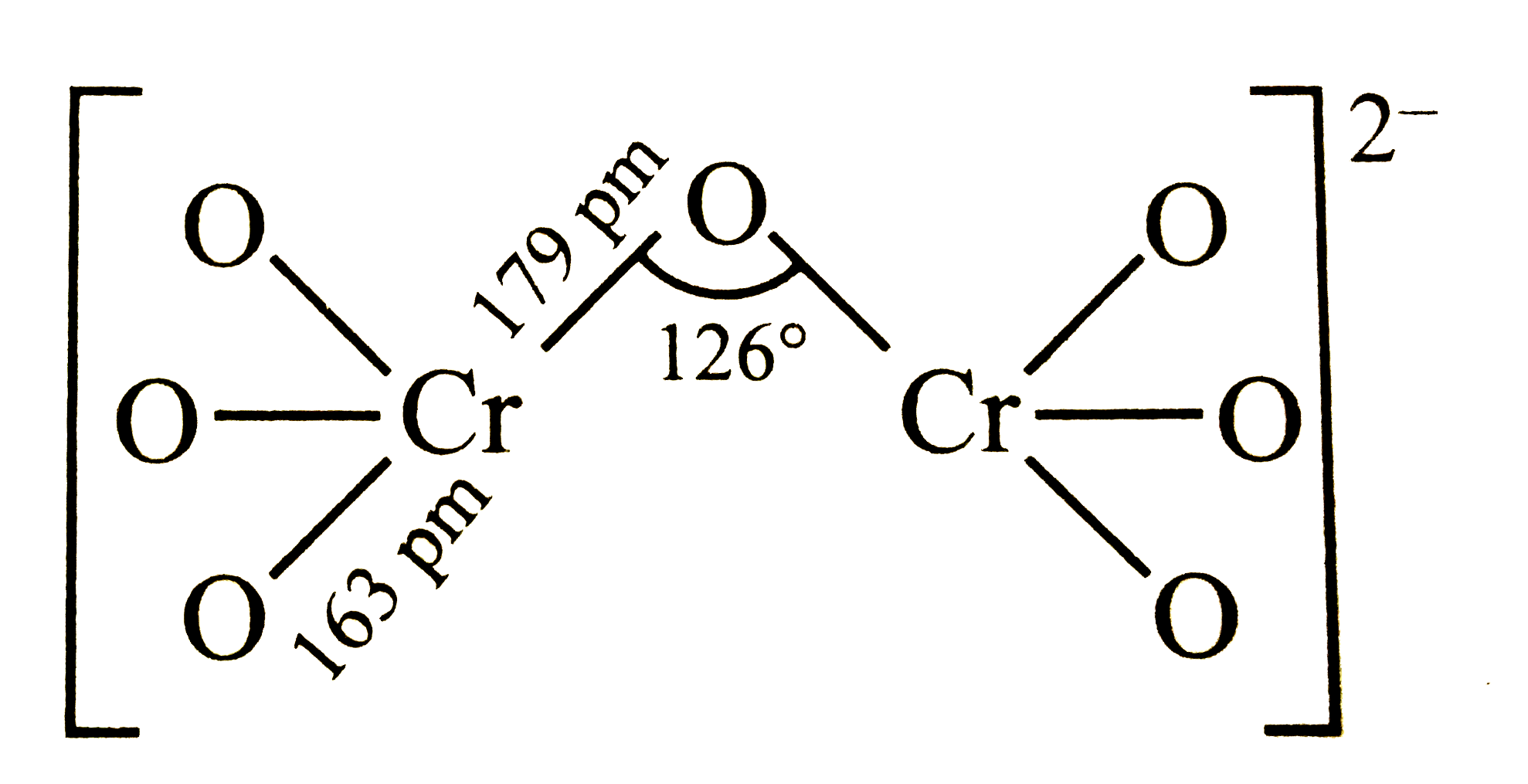
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THE D- AND F- BLOCK ELEMENTS
NCERT FINGERTIPS|Exercise The Lanthanoids|18 VideosTHE D- AND F- BLOCK ELEMENTS
NCERT FINGERTIPS|Exercise The Actinoids|6 VideosTHE D- AND F- BLOCK ELEMENTS
NCERT FINGERTIPS|Exercise General Properties Of The Transition Elements|39 VideosSURFACE CHEMISTRY
NCERT FINGERTIPS|Exercise Assertion And Reason|15 VideosTHE P-BLOCK ELEMENTS
NCERT FINGERTIPS|Exercise Assertion And Reason|15 Videos
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS-THE D- AND F- BLOCK ELEMENTS -Some Important Compounds Of Transition Elements
- Acidified potassium dichromate reacts with potassium iodide and oxidie...
Text Solution
|
- One mole of acidified K(2)Cr(2)O(7) on reaction with excess of KCl wil...
Text Solution
|
- What would happen when a solution of potassium chromate is treated wit...
Text Solution
|
- Identify the correct structure of dichromate ion.
Text Solution
|
- In the dichromate anion (Cr2O7^(2-))
Text Solution
|
- When MnO2 is fused with KOH and O2, what is the product formed and its...
Text Solution
|
- The equation 3MnO4^(2-)+4H^(+)to2MnO4^(-)+MnO2+2H2O represents
Text Solution
|
- Explain why does colour of KMnO(4) disappear when oxalic acid is added...
Text Solution
|
- Complete the given reactions. (A) 2MnO4^(-)+H2O+I^(-)to2ul((i))+2O...
Text Solution
|
- The number of moles of KMnO(4) that will be needed to react completely...
Text Solution
|
- Complete the given reaction. 2Mn^(2+)+5ul((i))+8H2Oto2ul((ii))+10ul...
Text Solution
|
- Which of the following is correct representation of reaction of acidif...
Text Solution
|
- Which of the following reactions is not correct ?
Text Solution
|
- A solution of KMnO4 is reduced to various products depending upon its ...
Text Solution
|
- In acidic medium, KMnO4 oxidises FeSO4 solution. Which of the followin...
Text Solution
|
- Complate the following reactions. (i) Cr2O7^(2-)+3SO2+2H^(+)to2Cr^(3...
Text Solution
|
- Complete the following reactions. (i) MnO4^(-)+2H2O+3e^(-)to""+4OH^...
Text Solution
|
- Fill the missing products in the following reactions. (i) 2MnI4^(-...
Text Solution
|
- When an oxide of manganese (P) is fused with KOH in the presence of an...
Text Solution
|
- A violet compound of manganese (P) decomposes on heating to liberate o...
Text Solution
|