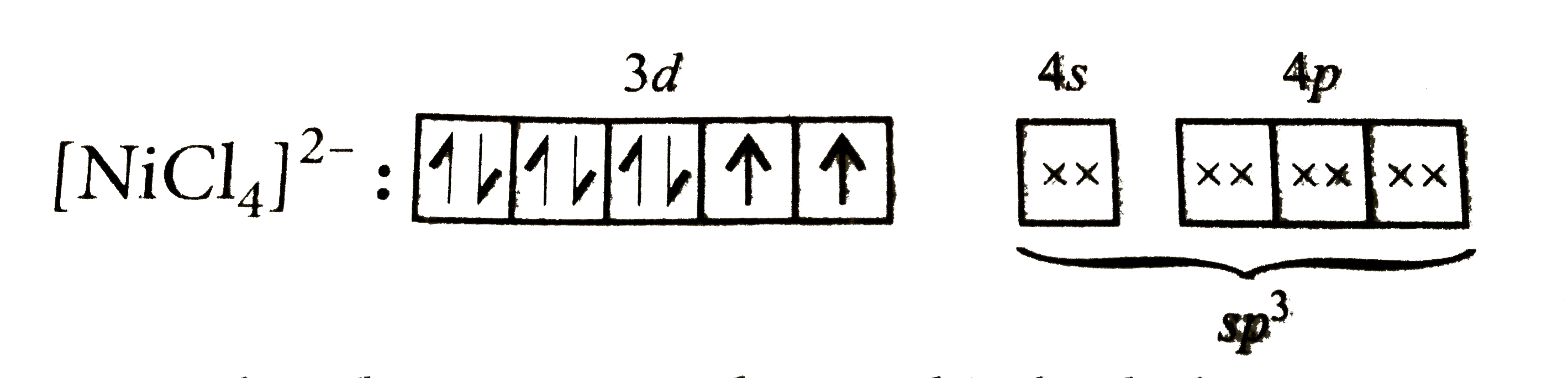
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
COORDINATION COMPOUNDS
NCERT FINGERTIPS|Exercise Bonding In Metal Carbonyls|5 VideosCOORDINATION COMPOUNDS
NCERT FINGERTIPS|Exercise Application Of Coordination Compounds|2 VideosCOORDINATION COMPOUNDS
NCERT FINGERTIPS|Exercise Isomerism In Coordination Compounds|16 VideosCHEMISTRY IN EVERYDAY LIFE
NCERT FINGERTIPS|Exercise Assertion And Reason|15 VideosELECTROCHEMISTRY
NCERT FINGERTIPS|Exercise Assertion And Reason|15 Videos
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS-COORDINATION COMPOUNDS -Bonding In Coordination Compounds
- Mark the incorrect statement .
Text Solution
|
- Which of the following facts about the complex [Cr(NH(3))(6)]Cl(3) is...
Text Solution
|
- Which of the following molecules has a regular tetrahedral shape?
Text Solution
|
- Mark the correct statements regarding the geometry of complex ions . ...
Text Solution
|
- Which of the following complex ion does not have 'd'-electrons in the ...
Text Solution
|
- Give reason for the statement [Ni(CN)(4)]^(2-) is diamagnetic while [N...
Text Solution
|
- The lowest value of paramagnetism is shown by
Text Solution
|
- Explain the following: (i) All the octahedral complexes of Ni^(2+)...
Text Solution
|
- Deduce the structures of [NiCl(4)]^(2-) and [Ni (CN)(4)]^(2-) consider...
Text Solution
|
- Which of the following has largest paramagnetism ?
Text Solution
|
- Using valence bond theory , the complex [Cr(H(2)O)(6)]^(3+) can be des...
Text Solution
|
- Which of the following descriptions about [FeCl(6)]^(4-) is correct ab...
Text Solution
|
- When excess of ammonia is added to CuSO4 solution, the deep blue colou...
Text Solution
|
- Pick out the correct statement with respect to [Mn(CN)(6)]^(3-):
Text Solution
|
- Among the following the compound that is both paramagnetic and coloure...
Text Solution
|
- The spin only magnetic moment value of Cr(CO)(6) is
Text Solution
|
- Which of the following complexes will show maximum paramagnetism ?
Text Solution
|
- Match the column I with column II and mark the appropriate choice .
Text Solution
|
- Electronic configuration of [Cu(NH(3))(6)]^(2+) on the basis of crysta...
Text Solution
|
- Which of the following energy level diagram for [FeF(6)]^(3-) is corre...
Text Solution
|