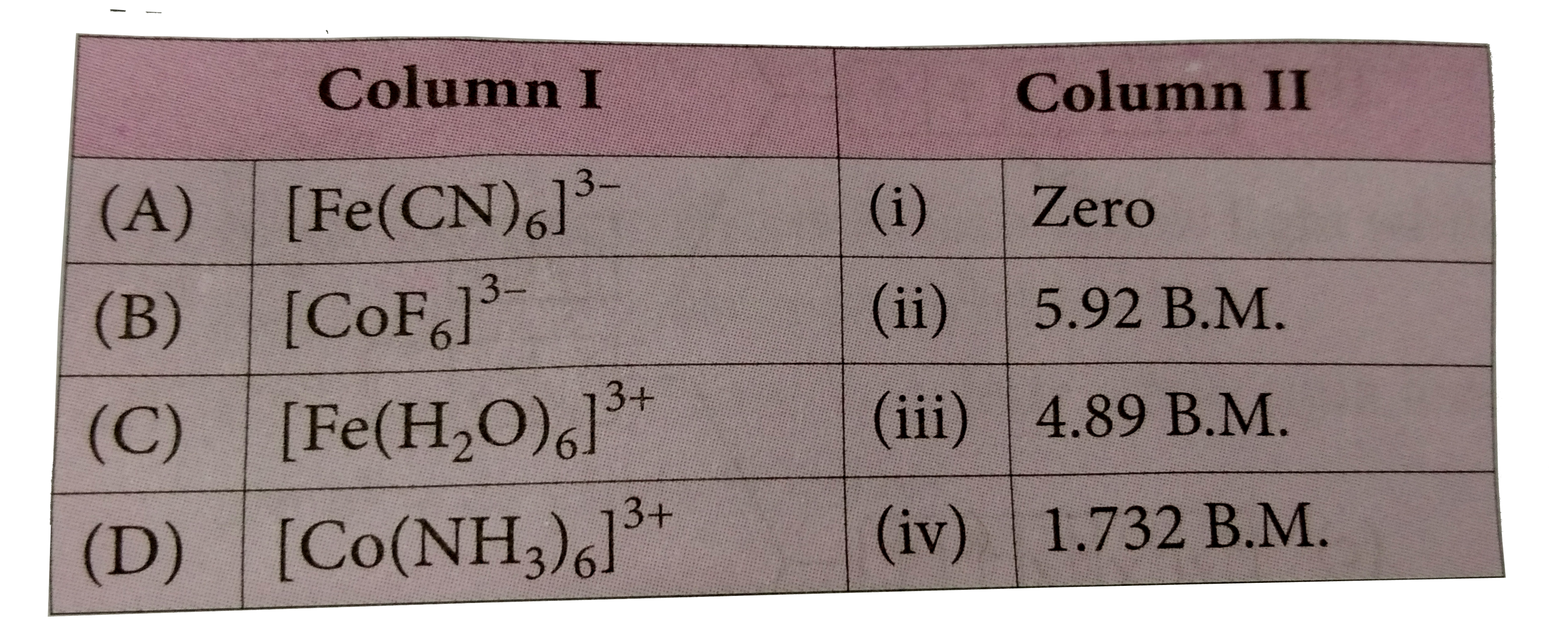A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
COORDINATION COMPOUNDS
NCERT FINGERTIPS|Exercise Bonding In Metal Carbonyls|5 VideosCOORDINATION COMPOUNDS
NCERT FINGERTIPS|Exercise Application Of Coordination Compounds|2 VideosCOORDINATION COMPOUNDS
NCERT FINGERTIPS|Exercise Isomerism In Coordination Compounds|16 VideosCHEMISTRY IN EVERYDAY LIFE
NCERT FINGERTIPS|Exercise Assertion And Reason|15 VideosELECTROCHEMISTRY
NCERT FINGERTIPS|Exercise Assertion And Reason|15 Videos
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS-COORDINATION COMPOUNDS -Bonding In Coordination Compounds
- The spin only magnetic moment value of Cr(CO)(6) is
Text Solution
|
- Which of the following complexes will show maximum paramagnetism ?
Text Solution
|
- Match the column I with column II and mark the appropriate choice .
Text Solution
|
- Electronic configuration of [Cu(NH(3))(6)]^(2+) on the basis of crysta...
Text Solution
|
- Which of the following energy level diagram for [FeF(6)]^(3-) is corre...
Text Solution
|
- In [NiCl(4)]^(2-) ,the number of unparied electron is
Text Solution
|
- Which of the following shell from an octahedral complex
Text Solution
|
- The value of 'spin only' magnetic moment for one of the following conf...
Text Solution
|
- Which of the following statements is/are correct ? (i) In octahedra...
Text Solution
|
- [FeF(6)]^(3-) is paramagnetic due to presence of unpaired electrons in...
Text Solution
|
- [Co(Cr(2)O(4))(3)]^(3-) is a
Text Solution
|
- What are the correct oxidation state , coordination number , configura...
Text Solution
|
- Which of the following statements is correct about [Co(H(2)O)(6)]^(2+)...
Text Solution
|
- The increasing order of the crystal field splitting power of some comm...
Text Solution
|
- What is crystal field splitting energy? How does the magnitude of tria...
Text Solution
|
- Why are low spin tetrahedral complexes not formed ?
Text Solution
|
- A substance appears coloured because
Text Solution
|
- Match the complex ions given in column I with their colour given in co...
Text Solution
|
- CuSO(4).5H(2)O is blue in colour while CuSO(4) is colourless. Why ?
Text Solution
|
- [Fe(CN)(6)]^(4-) and [Fe(H(2)O)(6)]^(2+) are of different colours in d...
Text Solution
|