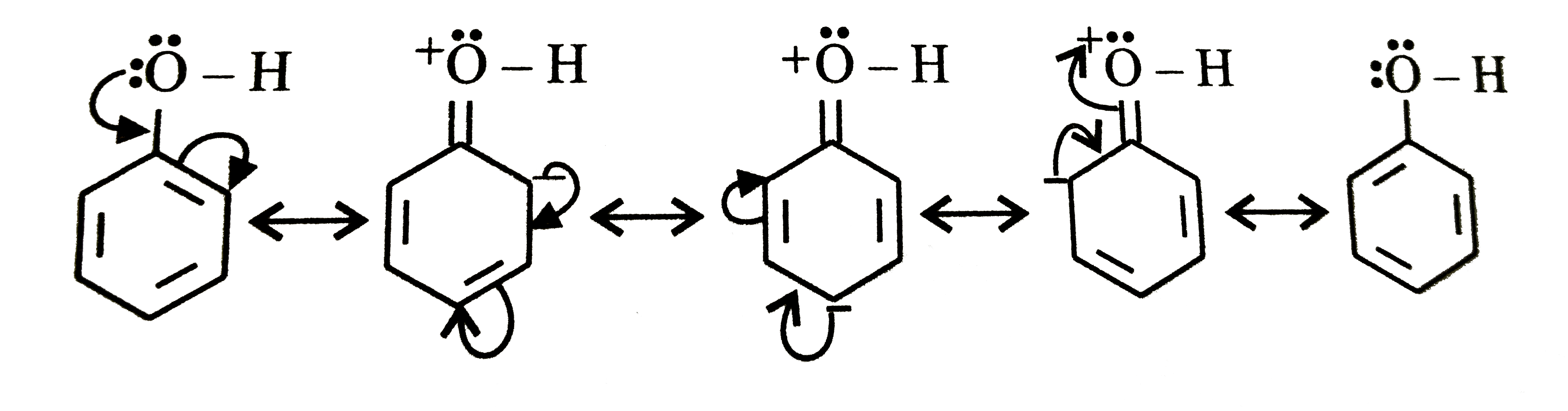
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ALCOHOLS,PHENOLS AND ETHERS
NCERT FINGERTIPS|Exercise Some Commercially Important Alcohols|4 VideosALCOHOLS,PHENOLS AND ETHERS
NCERT FINGERTIPS|Exercise Ethers|17 VideosALCOHOLS,PHENOLS AND ETHERS
NCERT FINGERTIPS|Exercise Structures Of Functional Groups|2 VideosALDEHYDES, KETONES AND CARBOXYLIC ACIDS
NCERT FINGERTIPS|Exercise Assertion And Reason|15 Videos
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS-ALCOHOLS,PHENOLS AND ETHERS-Alcohols And Phenols
- Which of the following is not true in case of reaction with heated cop...
Text Solution
|
- When tertiary butyl alcohol is passed over reduced copper, the reactio...
Text Solution
|
- Which of the following reagents can be used to oxidise primary alcohol...
Text Solution
|
- Which of the following compounds will be most easily attacked by an el...
Text Solution
|
- Out of phenol and benzene, which can be more easily nitrated ?
Text Solution
|
- Picric acid is a yellow coloured compound. Its chemical name is
Text Solution
|
- When phenol in treated with excess bromine water, it gives
Text Solution
|
- The structure of the compound that gives a tribromo derivative on trea...
Text Solution
|
- The major product obtained on interaction of phenol with sodium hydrox...
Text Solution
|
- The reaction between phenol and chloroform in the presence of aqueous ...
Text Solution
|
- Conversion of phenol to salicylic acid and to salicyaldehyde are known...
Text Solution
|
- In this given reactions, X and Y are respectively
Text Solution
|
- Identify the final product of the reaction sequence .
Text Solution
|
- Benzoquinone is prepared by reaction of phenol with
Text Solution
|
- Which of the following statements is correct ?
Text Solution
|
- p-cresol reacts with chloroform in alkaline medium to give the compoun...
Text Solution
|
- A primary alcohol, C(3)H(8)O(W) on heating with sulphuric acid undergo...
Text Solution
|
- Match the column I with column II and mark the appropriate choice .
Text Solution
|
- Consider the following reaction Phenol overset(Zn)underset("dust")ra...
Text Solution
|
- Match the column I with column II and mark the appropriate choice .
Text Solution
|