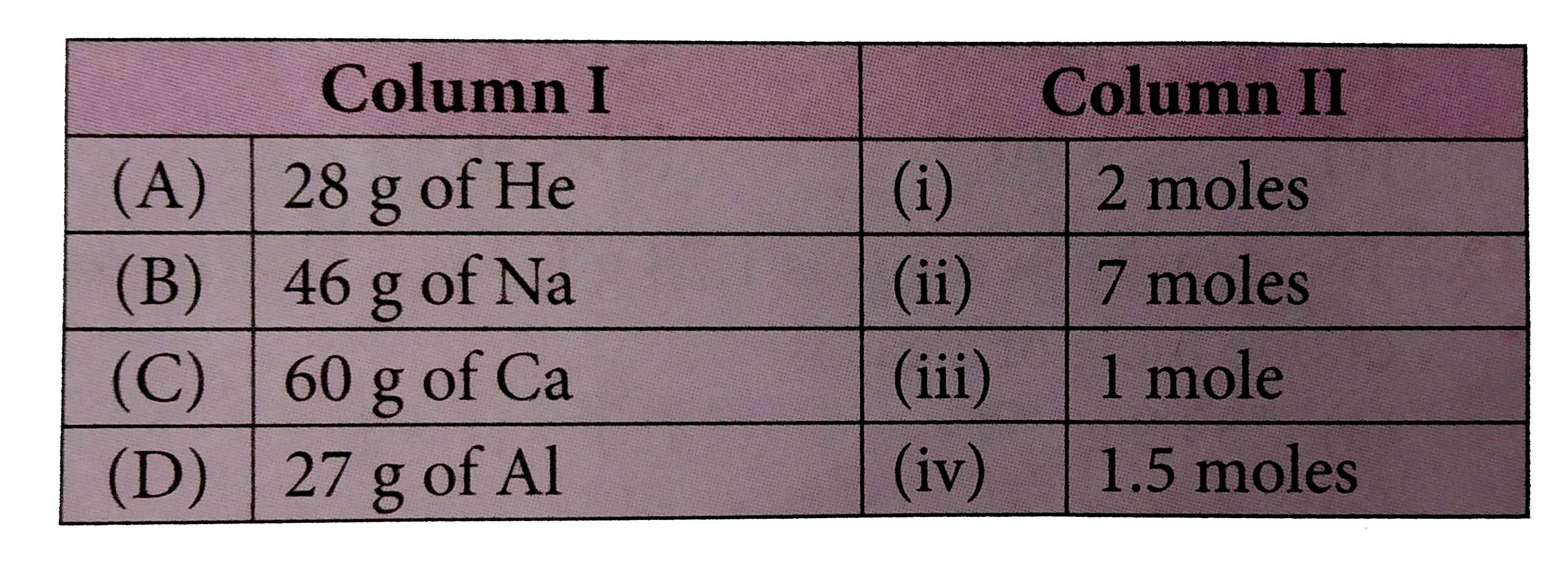
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
SOME BASIC CONCEPTS OF CHEMISTRY
NCERT FINGERTIPS|Exercise Percentage Composition|9 VideosSOME BASIC CONCEPTS OF CHEMISTRY
NCERT FINGERTIPS|Exercise Stoichiometry And Stoichiometry Calculations|28 VideosSOME BASIC CONCEPTS OF CHEMISTRY
NCERT FINGERTIPS|Exercise Atomic And Molecular Masses|6 VideosREDOX REACTIONS
NCERT FINGERTIPS|Exercise Assertion And Reason|15 VideosSTATES OF MATTER
NCERT FINGERTIPS|Exercise Assertion And Reason|15 Videos
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS-SOME BASIC CONCEPTS OF CHEMISTRY -Mole Concept And Molecular Masses
- The measured density at NTP of He is 0.1784 g L^(-1). Calculate the we...
Text Solution
|
- Which of the following gases will have least volume if 10g of each gas...
Text Solution
|
- How many number of molecules and atoms respectively are present in 2.8...
Text Solution
|
- Total number of atoms present in 34 g of NH3 is
Text Solution
|
- What will be the mass of 100 atoms of hydrogen?
Text Solution
|
- How many atoms in total are present in 1kg of sugar?
Text Solution
|
- 1.4 moles of phosphorus trichloride are present in a sample. How many ...
Text Solution
|
- What will be the standard molar volume of He, if its density is 0.1784...
Text Solution
|
- In a mixture of gases, the volume content of a gas is 0.06% at STP. Ca...
Text Solution
|
- What will be the weight of CO having the same number of oxygen atoms a...
Text Solution
|
- Match the mass of elements given in coloumn I with the no. of moles gi...
Text Solution
|
- Calculate the number of aluminium ions present in 0.051 g of aluminium...
Text Solution
|
- Which of the following correctly represents 180 g of water ? 5 mole...
Text Solution
|
- How many oxygen atoms will be present in 88 g of CO2 ?
Text Solution
|
- Calculate the total number of electrons present in 1.6 g of methane
Text Solution
|
- A mixture having 2 g of hydrogen and 32 oxygen occupies how much volum...
Text Solution
|
- One atom of an element weight 3.32xx10^(-25) g. How many number of gra...
Text Solution
|
- Fill in the blanks by choosing the correct option.
Text Solution
|
- The mass of one mole of a substance in grams is called its
Text Solution
|
- How much copper is present in 50 g of CuSO4
Text Solution
|