A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SOME BASIC CONCEPTS OF CHEMISTRY
NCERT FINGERTIPS|Exercise Stoichiometry And Stoichiometry Calculations|28 VideosSOME BASIC CONCEPTS OF CHEMISTRY
NCERT FINGERTIPS|Exercise MCQ|1 VideosSOME BASIC CONCEPTS OF CHEMISTRY
NCERT FINGERTIPS|Exercise Mole Concept And Molecular Masses|24 VideosREDOX REACTIONS
NCERT FINGERTIPS|Exercise Assertion And Reason|15 VideosSTATES OF MATTER
NCERT FINGERTIPS|Exercise Assertion And Reason|15 Videos
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS-SOME BASIC CONCEPTS OF CHEMISTRY -Percentage Composition
- 0.48 g of a sample of a compound containing boron and oxygen contains ...
Text Solution
|
- A compound of magnesium contains 21.9% magnesium, 27.8% phosphorus and...
Text Solution
|
- A compound contains two elements 'X' and 'Y' in the ratio of 50% each....
Text Solution
|
- The empirical formula of a compound is CH2O2. What could be its molecu...
Text Solution
|
- A gas has molecular formula (CH)n. If vapour density of the gas is 39...
Text Solution
|
- Determine the molecular formula of an oxide of iron in which the mass ...
Text Solution
|
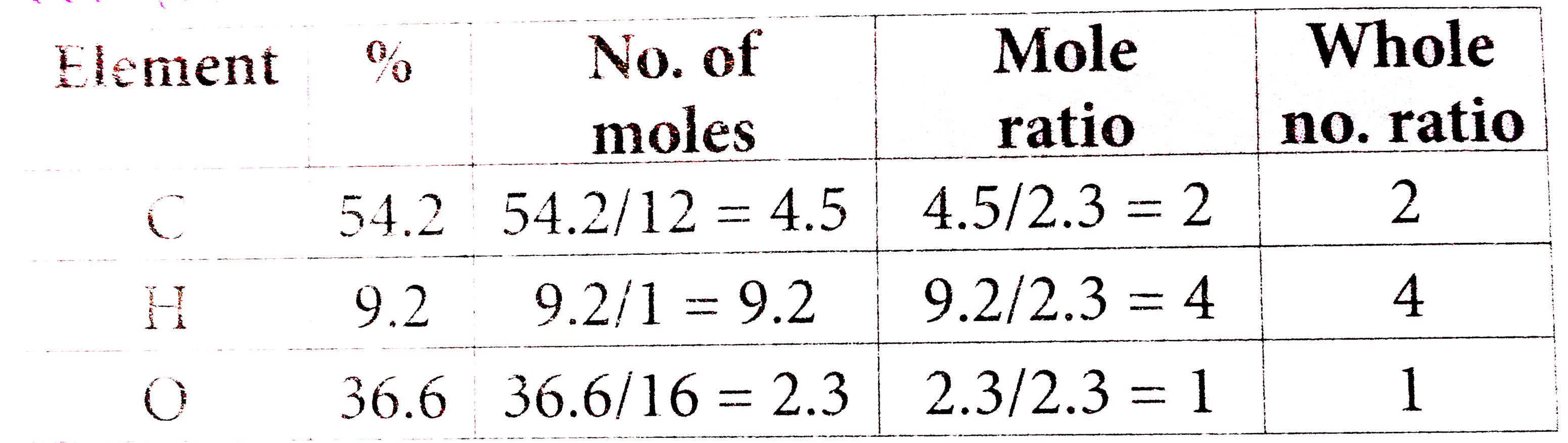
- An organic compound on analysis gave C=54.2%, H=9.2% by mass. Its empi...
Text Solution
|
- The relative number of mass of elements, 'X' and 'Y' in a compound is ...
Text Solution
|
- Two elements 'P' and 'Q' combine to form a compound. Atomic mass of 'p...
Text Solution
|