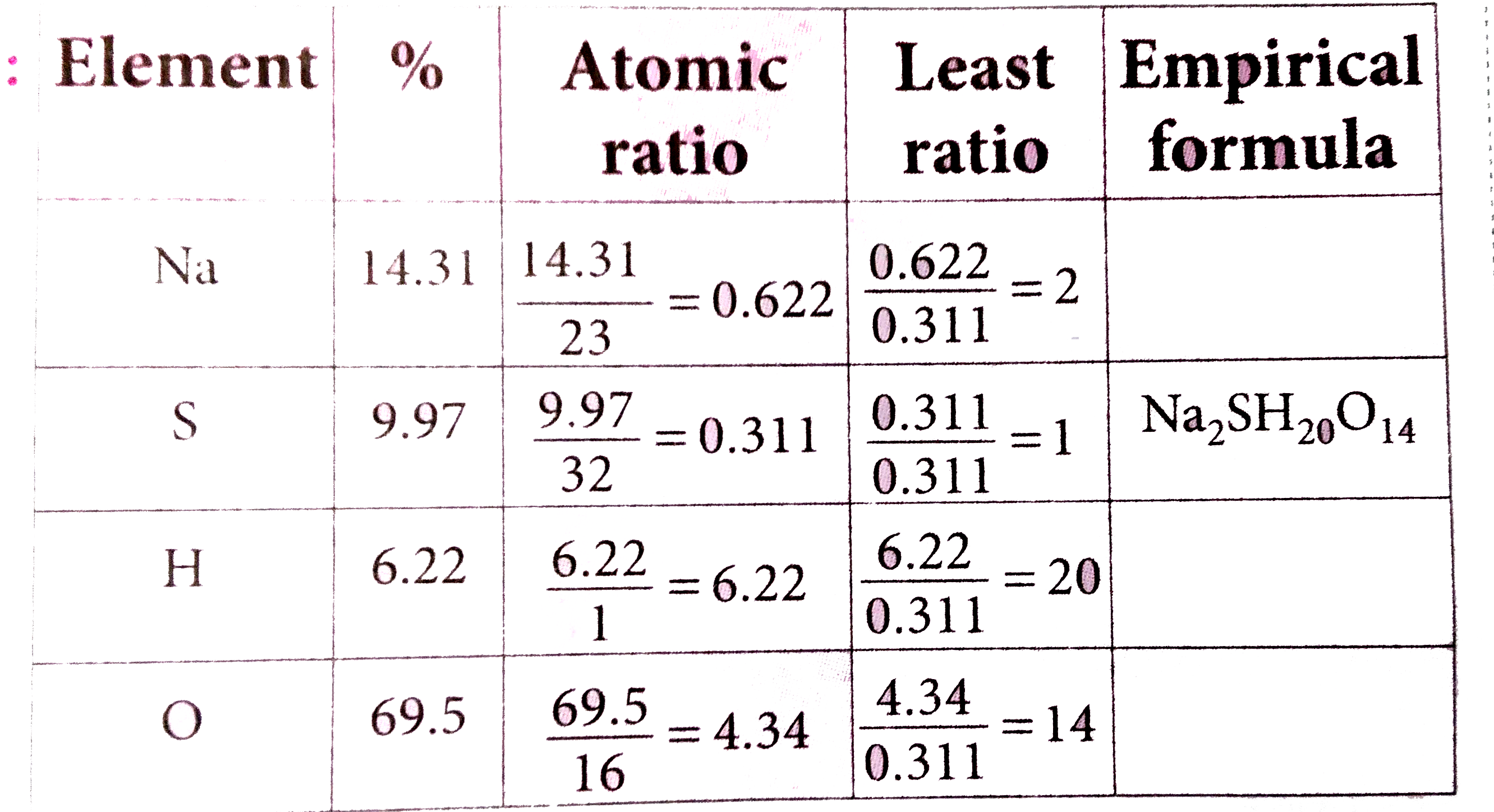A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
SOME BASIC CONCEPTS OF CHEMISTRY
NCERT FINGERTIPS|Exercise NCERT Exemplar|11 VideosSOME BASIC CONCEPTS OF CHEMISTRY
NCERT FINGERTIPS|Exercise Assertion And Reason|10 VideosSOME BASIC CONCEPTS OF CHEMISTRY
NCERT FINGERTIPS|Exercise MCQ|1 VideosREDOX REACTIONS
NCERT FINGERTIPS|Exercise Assertion And Reason|15 VideosSTATES OF MATTER
NCERT FINGERTIPS|Exercise Assertion And Reason|15 Videos
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS-SOME BASIC CONCEPTS OF CHEMISTRY -Higher Order Thinking Skills
- 45.4L of dinitrogen reacted with 22.7L of dioxygen and 45.4 L of nitro...
Text Solution
|
- Hydrogen gas is prepared in the laboratory by reacting dilute HCl with...
Text Solution
|
- Chemical reactions involve interaction of atoms and molecules. A large...
Text Solution
|
- Chemical reactions involve interaction of atoms and molecules. A large...
Text Solution
|
- The total charge (coulombs) required for complete electrolysis is
Text Solution
|
- A compound on analysis was found to contain the following composition ...
Text Solution
|
- The reactant which is entirely consumed in reaction is known as limiti...
Text Solution
|
- The density of 3 molal solution of NaOH is 1.110g mL^(-1). Calculate t...
Text Solution
|
- 1 L of 0.1 M NaOH, 1 L of 0.2 M KOH, and 2 L of 0.05 M Ba (OH)(2) are ...
Text Solution
|