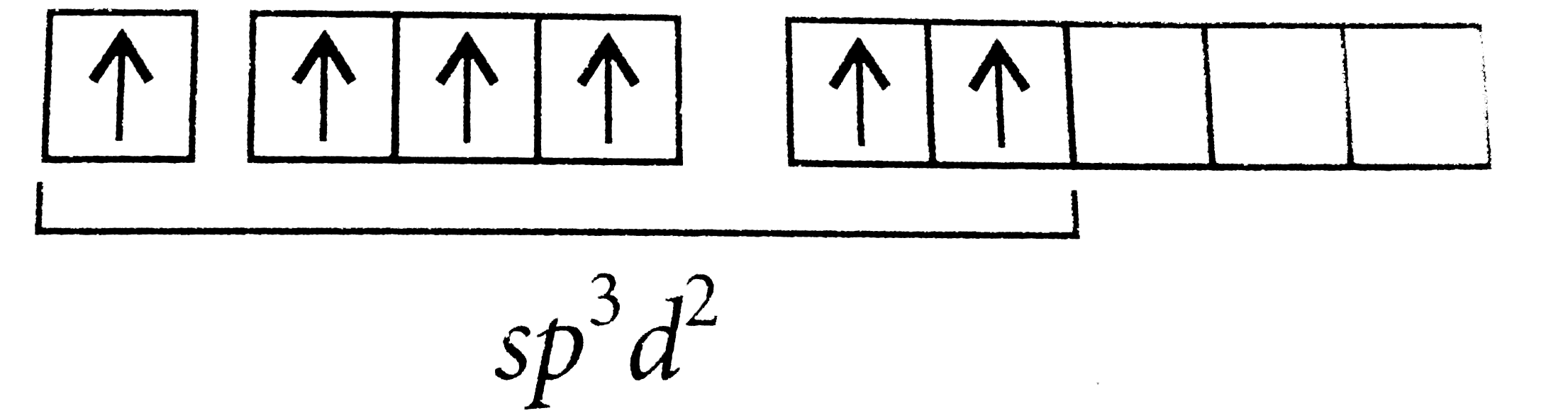A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING & MOLECULAR STRUCTURE
NCERT FINGERTIPS|Exercise Molecular Orbital Theory|18 VideosCHEMICAL BONDING & MOLECULAR STRUCTURE
NCERT FINGERTIPS|Exercise Bonding In Some Homonuclear Diatomic Molecules|4 VideosCHEMICAL BONDING & MOLECULAR STRUCTURE
NCERT FINGERTIPS|Exercise Valence Bond Theory|7 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY
NCERT FINGERTIPS|Exercise Assertion And Reason|15 Videos
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS-CHEMICAL BONDING & MOLECULAR STRUCTURE-Hybridisation
- Which of the following statements is true about hybridisation ?
Text Solution
|
- In which of the following the central atom does not use sp^(2) hybrid ...
Text Solution
|
- carbon in carbon dioxide is
Text Solution
|
- Which type of hybridisation is shown by carbon atoms from left to righ...
Text Solution
|
- On hybridisation of one s and three p-orbitals, we get
Text Solution
|
- Among the following molecules : SO(2),SF(4) ,CIF(3) ,BrF(5) , and XeF(...
Text Solution
|
- Which of the following does not involves dsp^(2)-hybridisation and are...
Text Solution
|
- Which of the following pairs of species are isostructural ?
Text Solution
|
- The increasing d-character in hybridisation of Xe in XeF(2), XeF(4), X...
Text Solution
|
- What is the hybrid state of carbon in ethyne, graphite and diamond ?
Text Solution
|
- Given below is the bond angle in various types of hybridisation. Mark...
Text Solution
|
- Which of the following molecules possess sp,sp^2 and sp^3 hybridized C...
Text Solution
|
- Match the column I with column II and mark the appropriate choice. {...
Text Solution
|
- In formation of ethene, the bond formation between s and p- orbitals t...
Text Solution
|
- The ground state electronic configuration of S is 3s^(2) 3 p^(4) . Ho...
Text Solution
|