A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING & MOLECULAR STRUCTURE
NCERT FINGERTIPS|Exercise NCERT Exemplar|15 VideosCHEMICAL BONDING & MOLECULAR STRUCTURE
NCERT FINGERTIPS|Exercise Assertion And Reason|15 VideosCHEMICAL BONDING & MOLECULAR STRUCTURE
NCERT FINGERTIPS|Exercise Hydrogen Bonding|9 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY
NCERT FINGERTIPS|Exercise Assertion And Reason|15 Videos
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS-CHEMICAL BONDING & MOLECULAR STRUCTURE-Higher Order Thinking Skills
- NaCl((aq)) gives a white precipitate with AgNO(3) solution but C Cl(4)...
Text Solution
|
- The lattice energy of NaCl is 788 kJ mol^(-1) . This means that 788 kJ...
Text Solution
|
- The dipole moment of LiH is 1.964 xx10^(-29)C-m and the interatomic di...
Text Solution
|
- Which is the correct order of bond lengths P,Q and R in Hoverset(p)(...
Text Solution
|
- Consider the following molecules underset(I)(O(2)),O(2)underset(II)(...
Text Solution
|
- The relationship between the dissociation energy of N2 and N2^+ is
Text Solution
|
- Molecular shape of SF(4), CF(4) and XeF(4) are
Text Solution
|
- The AsF5 molecule is trigonal bipyramidal. The orbitals used by As for...
Text Solution
|
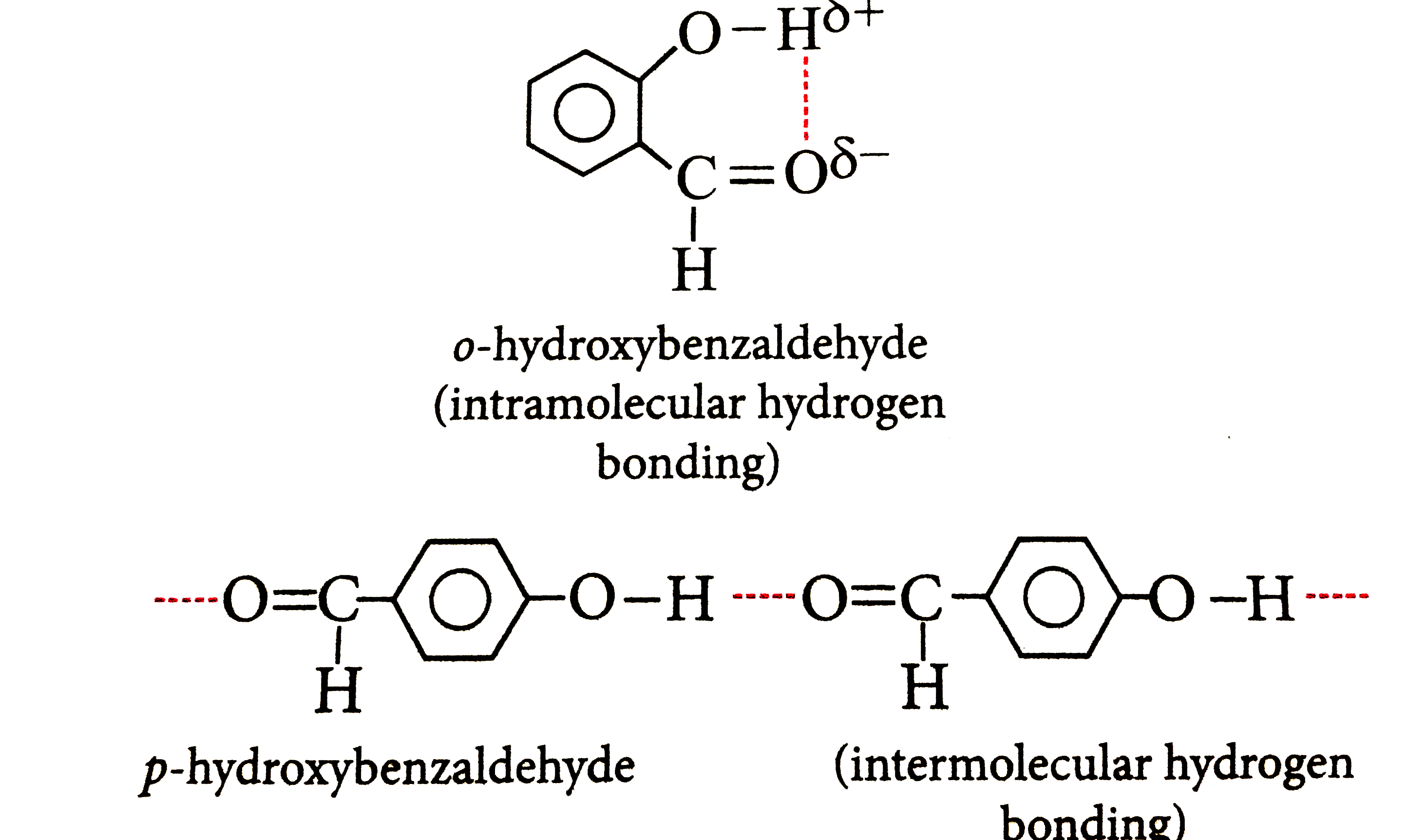
- Explain, why o-hydroxybenzaldehyde is a liquid at room temperature whi...
Text Solution
|
- Ethy1 alcohol (C(2)H(5)OH) has higher boiling point than dimethyl ethe...
Text Solution
|