A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS-THERMODYNAMICS-Applications
- Two litres of an ideal gas at a pressure of 10 atm expands isothermall...
Text Solution
|
- If the heat change at constant volume for decomposition of silver oxid...
Text Solution
|
- The enthalpy change of a reaction does not depend upon
Text Solution
|
- Consider the given diagram for 1 mole of a gas X and answer the follow...
Text Solution
|
- Which of the following relationships is not correct for the relation b...
Text Solution
|
- Two reactions are given below : (i) CO((g))+(1)/(2)O(2(g))rarrCO(2(...
Text Solution
|
- Consider the following reaction : CO((g))+(1)/(2)O(2(g)) rarr CO(2(g...
Text Solution
|
- Match the column I with column II and mark the appropriate choice.
Text Solution
|
- For combustion of 1 mole of benzene at 25^(@)C, the heat of reaction a...
Text Solution
|
- Reaction of methanol with dioxygen was carried out and DeltaU was foun...
Text Solution
|
- According to the first law of thermodynamics, DeltaU=q+w. In special c...
Text Solution
|
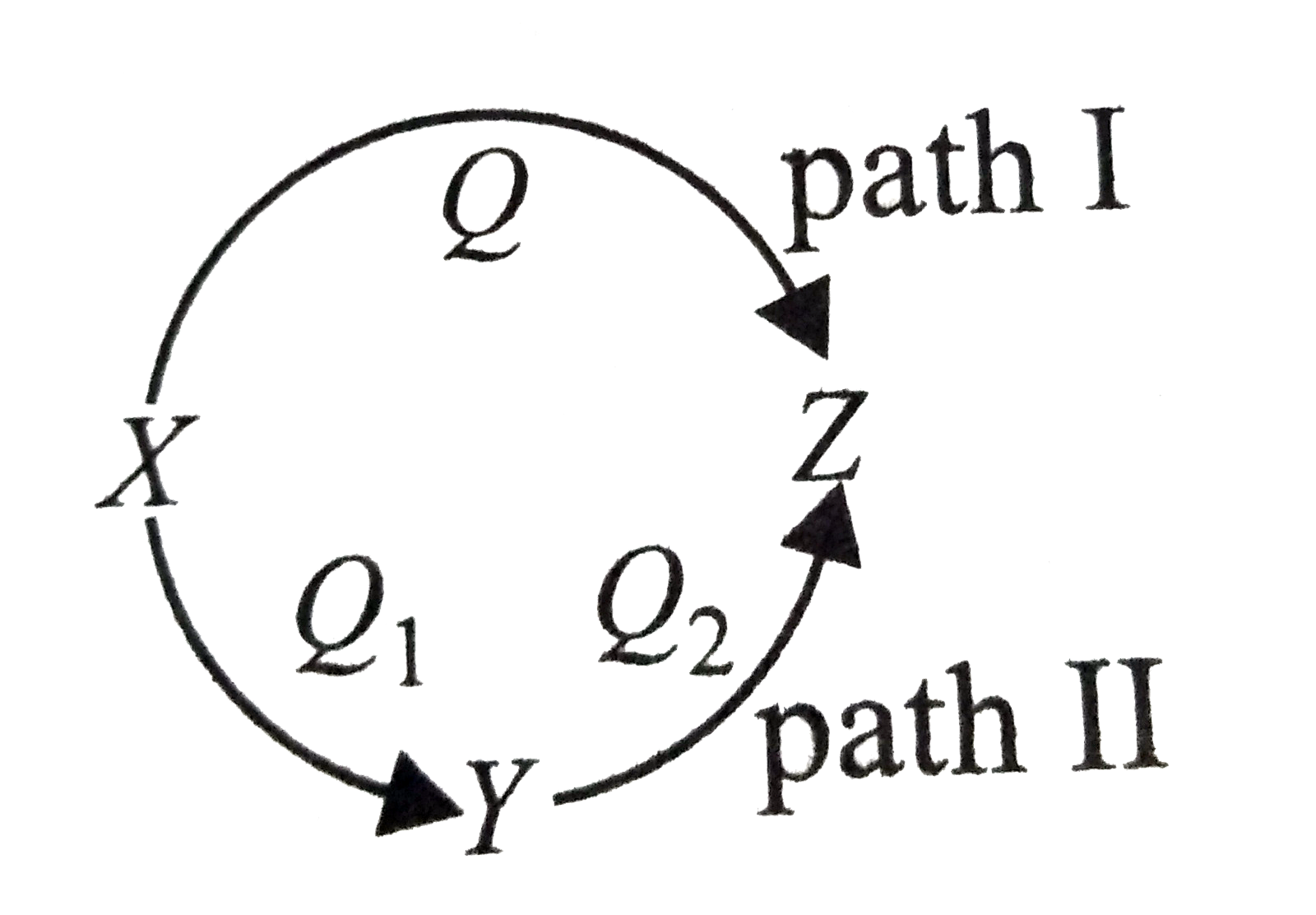
- A reaction proceeds through two paths I and II to convert X rarr Z. ...
Text Solution
|
- What will be the standard internal energy change for the reaction at 2...
Text Solution
|
- The value for DeltaU for the reversible isothermal evaporation of 90 ...
Text Solution
|
- A system changes from state X to Y with a change in internal energy me...
Text Solution
|
- What will be the change in internal energy when 12 kJ of work is done ...
Text Solution
|
- A system absorbs 50 kJ heat and does 20 kJ of work. What is the net ch...
Text Solution
|
- 200 joules of heat was supplied to a system at constant volume. It re...
Text Solution
|
- In thermodynamics, which one of the following properties is not an int...
Text Solution
|
- The molar heat capacity of water at constant pressure, C(p) is "75 J K...
Text Solution
|