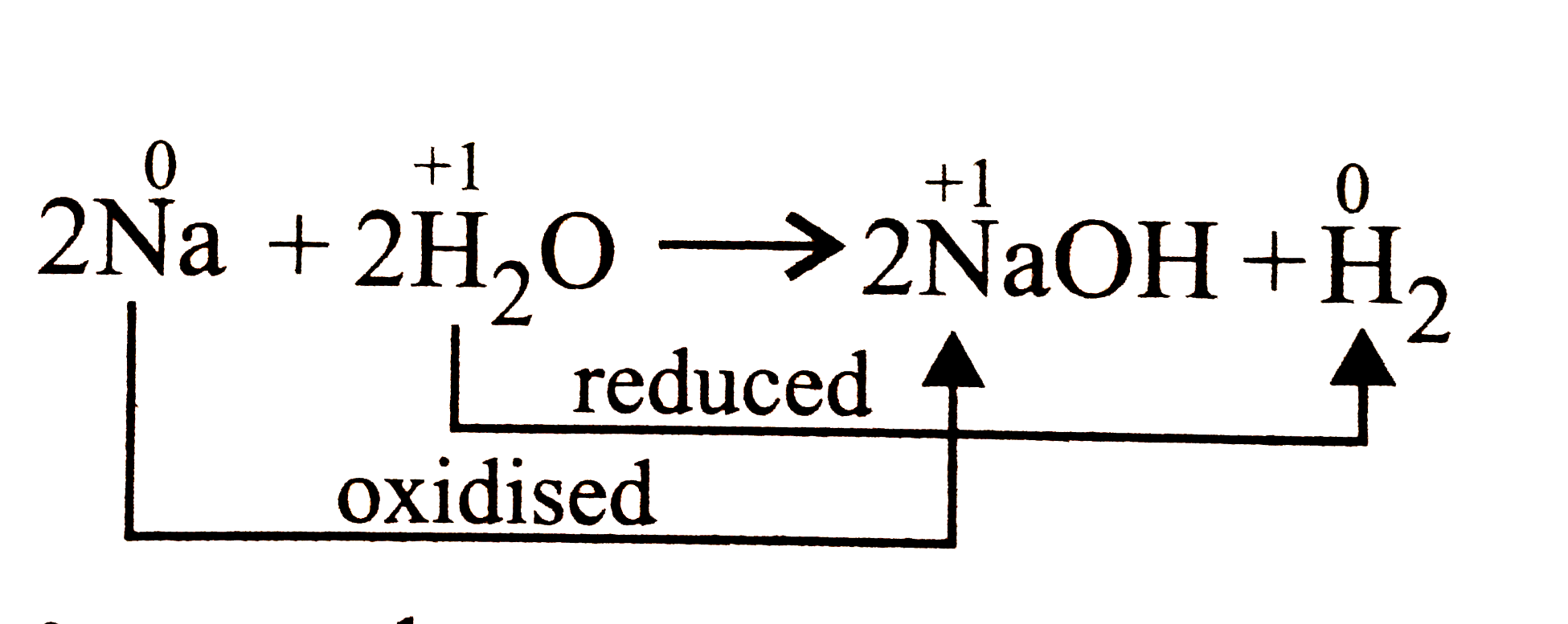A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
REDOX REACTIONS
NCERT FINGERTIPS|Exercise MCQs (REDOX REACTIONS AND ELECTRODE PROCESSES)|1 VideosREDOX REACTIONS
NCERT FINGERTIPS|Exercise Redox Reactions And Electrode Processes|22 VideosREDOX REACTIONS
NCERT FINGERTIPS|Exercise MCQs (OXIDATION NUMBER)|1 VideosPRACTICE PAPER 3
NCERT FINGERTIPS|Exercise Practice Paper 3|46 VideosSOME BASIC CONCEPTS OF CHEMISTRY
NCERT FINGERTIPS|Exercise Assertion And Reason|10 Videos
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS-REDOX REACTIONS-Oxidation Number
- Oxidation number of P in Ba(H(2)PO(2))(2) is
Text Solution
|
- Which of the following can act as oxidising as well as reducing agent?
Text Solution
|
- When a piece of sodium metal is dropped in water, hydrogen gas evolved...
Text Solution
|
- In the reaction, I(2)+2S(2)O(3)^(2-) rarr 2I^(-)+S(4)O(6)^(2-). Eq...
Text Solution
|
- In the reaction :Cl(2)+OH^(-)rarrCl^(-)+ClO(4)^(-)+H(2)O :-
Text Solution
|
- Consider the following reaction : HCHO+2[Ag(NH(3))(2)]^(+)+3OH^(-)rarr...
Text Solution
|
- Identify the compounds which are reduced and oxidised in the followin...
Text Solution
|
- Identify the oxidant and reductant in the following redox reaction: ...
Text Solution
|
- Indicate whether the following conversions represent an oxidation, a r...
Text Solution
|
- In which of the following reactions, the underlined substance has been...
Text Solution
|
- A compound contains atoms A, B and C. the oxidation number of A is +2,...
Text Solution
|
- Consider the following reactions, (I) 2Mn(2)O(7) rarr 4MnO(2) + 3O(2...
Text Solution
|
- Which of the following statements is correct regarding redox reactions...
Text Solution
|
- In the reacion , 3Br(2)+6CO(3)^(2-)+3H(2)Orarr5Br^(-)+BrO(3)^(-)+6HC...
Text Solution
|
- Given below is a redox reaction. Which of the following types the rea...
Text Solution
|
- Identify the oxidant the reductant in the following reaction. Cl(2)...
Text Solution
|
- Which of the following is a disproportionation reaction?
Text Solution
|
- Match the column I with column II with the type of reaction and mark t...
Text Solution
|
- Which of the following is not an example of disproportionation reactio...
Text Solution
|
- White phosphorus reacts with caustic soda to form PH(3) and NaH(2) PO(...
Text Solution
|