A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
REDOX REACTIONS
NCERT FINGERTIPS|Exercise MCQs (REDOX REACTIONS AND ELECTRODE PROCESSES)|1 VideosREDOX REACTIONS
NCERT FINGERTIPS|Exercise Redox Reactions And Electrode Processes|22 VideosREDOX REACTIONS
NCERT FINGERTIPS|Exercise MCQs (OXIDATION NUMBER)|1 VideosPRACTICE PAPER 3
NCERT FINGERTIPS|Exercise Practice Paper 3|46 VideosSOME BASIC CONCEPTS OF CHEMISTRY
NCERT FINGERTIPS|Exercise Assertion And Reason|10 Videos
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS-REDOX REACTIONS-Oxidation Number
- Identify the oxidant the reductant in the following reaction. Cl(2)...
Text Solution
|
- Which of the following is a disproportionation reaction?
Text Solution
|
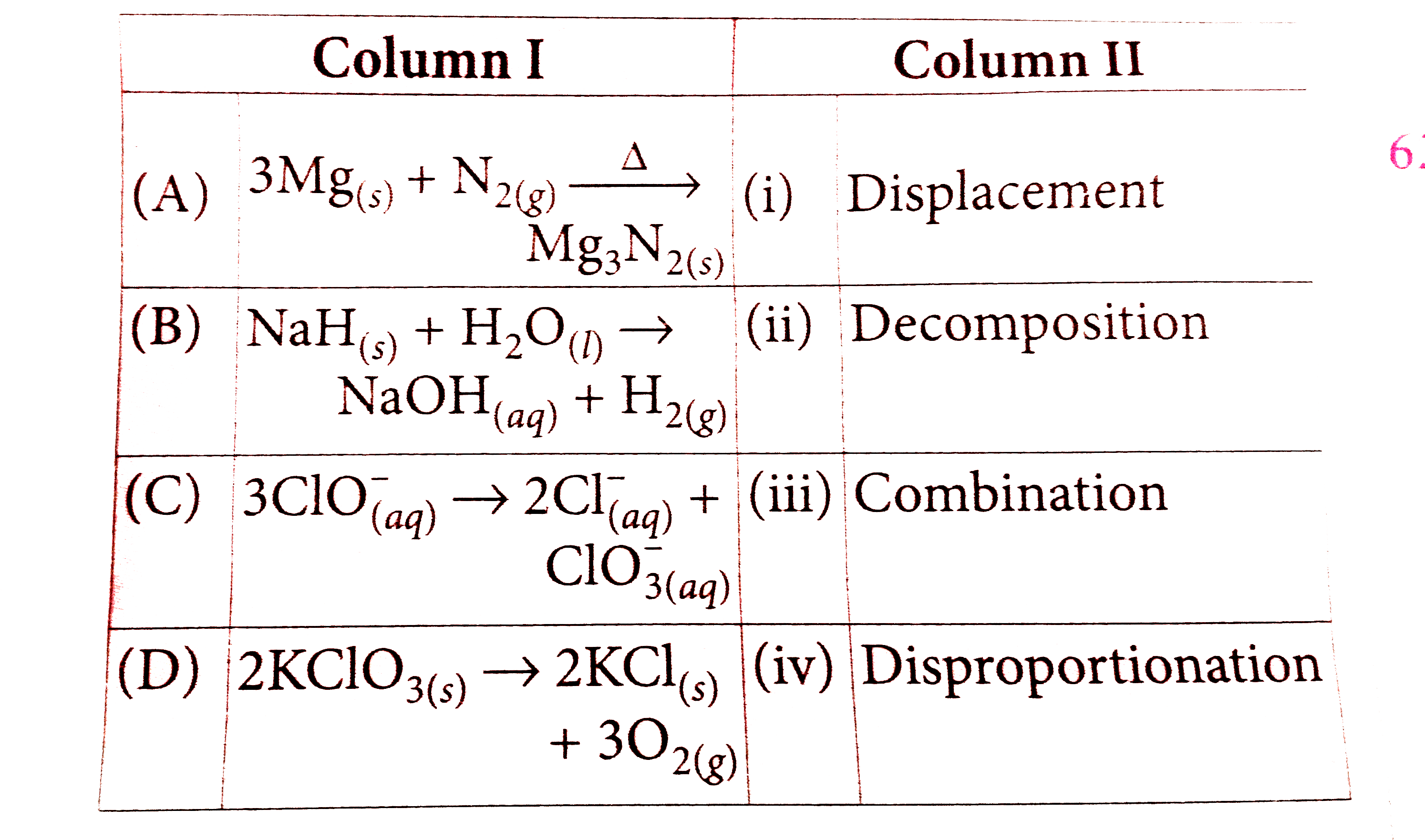
- Match the column I with column II with the type of reaction and mark t...
Text Solution
|
- Which of the following is not an example of disproportionation reactio...
Text Solution
|
- White phosphorus reacts with caustic soda to form PH(3) and NaH(2) PO(...
Text Solution
|
- What is the oxidation number of carbon in C(3)O(2) ( carbon suboxide )...
Text Solution
|
- The oxidation number of Cr in CrO(5) which has the following structur...
Text Solution
|
- In the conversion of Br2 to BrO3^(-) , the oxidation state of Br chan...
Text Solution
|
- Permanganate (VII) ion, MnO(4)^(-) oxidises I^(-) ion to I(2) and giv...
Text Solution
|
- Choose correct statements (s) regarding the following reactions. Cr(...
Text Solution
|
- The Mn^(3+) ion is unstable in solution and undergoes disproportionati...
Text Solution
|
- The number of moles of KMnO(4) reduced by 1 "mol of" KI in alkaline me...
Text Solution
|
- Balance the following equation by oxidation number method: K(2)Cr(2)...
Text Solution
|
- Which will be the value of x, y and z in the following equaton. xI(2...
Text Solution
|
- The number of electrons involved in the conversion of MnO(4)^(-) to Mn...
Text Solution
|
- The values of coefficients to balance the following reaction are Cr(...
Text Solution
|
- The stoichiometric constants for the reaction pCu+qHNO(3) rarr rCu(NO...
Text Solution
|
- What is the correct representation of reaction occurring when HCl is h...
Text Solution
|
- When KMnO(4) is reduced with oxalic acid in acidic solution, the oxida...
Text Solution
|
- When a mananous salt is fused with a mixture of KNO(3) and and solid N...
Text Solution
|